Page 115 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 115
P1: GPB/GJP P2: FYK Final Pages
Encyclopedia of Physical Science and Technology EN005F-213 June 15, 2001 20:32
Electron Transfer Reactions 357
+
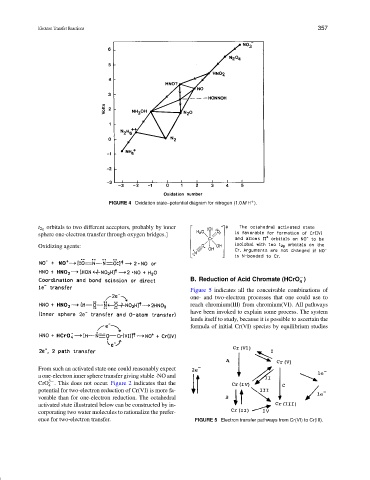
FIGURE 4 Oxidation state–potential diagram for nitrogen (1.0M H ).
t 2g orbitals to two different acceptors, probably by inner
sphere one-electron transfer through oxygen bridges.]
Oxidizing agents:
−
B. Reduction of Acid Chromate (HCrO )
4
Figure 5 indicates all the conceivable combinations of
one- and two-electron processes that one could use to
reach chromium(III) from chromium(VI). All pathways
have been invoked to explain some process. The system
lends itself to study, because it is possible to ascertain the
formula of initial Cr(VI) species by equilibrium studies
From such an activated state one could reasonably expect
a one-electron inner sphere transfer giving stable ·NO and
CrO . This does not occur. Figure 2 indicates that the
3−
4
potential for two-electron reduction of Cr(VI) is more fa-
vorable than for one-electron reduction. The octahedral
activated state illustrated below can be constructed by in-
corporating two water molecules to rationalize the prefer-
ence for two-electron transfer. FIGURE 5 Electron transfer pathways from Cr(VI) to Cr(III).