Page 185 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 185
P1: GTV Final Pages
Encyclopedia of Physical Science and Technology EN008H-970 June 29, 2001 16:46
Liquid Alkali Metals 665
◦
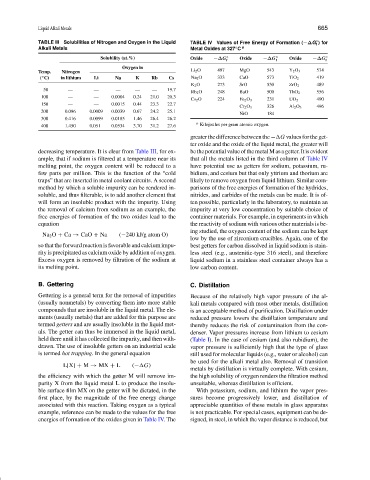
TABLE III Solubilities of Nitrogen and Oxygen in the Liquid TABLE IV Values of Free Energy of Formation (−∆G ) for
f
◦
Alkali Metals Metal Oxides at 327 C a
Solubility (at.%) Oxide −∆G ◦ f Oxide −∆G ◦ f Oxide −∆G ◦ f
Oxygen in
Temp. Nitrogen Li 2 O 497 MgO 543 Y 2 O 3 574
◦
( C) in lithium Li Na K Rb Cs Na 2 O 333 CaO 573 TiO 2 419
K 2 O 273 SrO 530 ZrO 2 489
50 — — — — — 19.7
Rb 2 O 248 BaO 500 ThO 2 556
100 — — 0.0004 0.24 21.0 20.3
Cs 2 O 224 Fe 2 O 3 231 UO 2 490
150 — — 0.0015 0.44 23.3 22.7
Cr 2 O 3 326 Al 2 O 3 496
200 0.086 0.0009 0.0039 0.67 24.2 25.1
NiO 184
300 0.416 0.0099 0.0185 1.46 26.4 26.2
a
400 1.450 0.051 0.0534 3.70 31.2 27.6 Kilojoules per gram atomic oxygen.
greater the difference between the − G values for the get-
ter oxide and the oxide of the liquid metal, the greater will
decreasing temperature. It is clear from Table III, for ex- bethepotentialvalueofthemetalMasagetter.Itisevident
ample, that if sodium is filtered at a temperature near its that all the metals listed in the third column of Table IV
melting point, the oxygen content will be reduced to a have potential use as getters for sodium, potassium, ru-
few parts per million. This is the function of the “cold bidium, and cesium but that only yttrium and thorium are
traps” that are inserted in metal coolant circuits. A second likely to remove oxygen from liquid lithium. Similar com-
method by which a soluble impurity can be rendered in- parisons of the free energies of formation of the hydrides,
soluble, and thus filterable, is to add another element that nitrides, and carbides of the metals can be made. It is of-
will form an insoluble product with the impurity. Using ten possible, particularly in the laboratory, to maintain an
the removal of calcium from sodium as an example, the impurity at very low concentration by suitable choice of
free energies of formation of the two oxides lead to the container materials. For example, in experiments in which
equation the reactivity of sodium with various other materials is be-
ing studied, the oxygen content of the sodium can be kept
Na 2 O + Ca → CaO + Na (−240 kJ/g atom O)
low by the use of zirconium crucibles. Again, one of the
so that the forward reaction is favorable and calcium impu- best getters for carbon dissolved in liquid sodium is stain-
rity is precipitated as calcium oxide by addition of oxygen. less steel (e.g., austenitic-type 316 steel), and therefore
Excess oxygen is removed by filtration of the sodium at liquid sodium in a stainless steel container always has a
its melting point. low carbon content.
B. Gettering C. Distillation
Gettering is a general term for the removal of impurities Because of the relatively high vapor pressure of the al-
(usually nonmetals) by converting them into more stable kali metals compared with most other metals, distillation
compounds that are insoluble in the liquid metal. The ele- is an acceptable method of purification. Distillation under
ments (usually metals) that are added for this purpose are reduced pressure lowers the distillation temperature and
termed getters and are usually insoluble in the liquid met- thereby reduces the risk of contamination from the con-
als. The getter can thus be immersed in the liquid metal, denser. Vapor pressures increase from lithium to cesium
held there until it has collected the impurity, and then with- (Table I). In the case of cesium (and also rubidium), the
drawn. The use of insoluble getters on an industrial scale vapor pressure is sufficiently high that the type of glass
is termed hot trapping. In the general equation still used for molecular liquids (e.g., water or alcohol) can
be used for the alkali metal also. Removal of transition
L[X] + M → MX + L (− G)
metals by distillation is virtually complete. With cesium,
the efficiency with which the getter M will remove im- the high solubility of oxygen renders the filtration method
purity X from the liquid metal L to produce the insolu- unsuitable, whereas distillation is efficient.
ble surface film MX on the getter will be dictated, in the With potassium, sodium, and lithium the vapor pres-
first place, by the magnitude of the free energy change sures become progressively lower, and distillation of
associated with this reaction. Taking oxygen as a typical appreciable quantities of these metals in glass apparatus
example, reference can be made to the values for the free is not practicable. For special cases, equipment can be de-
energies of formation of the oxides given in Table IV. The signed, in steel, in which the vapor distance is reduced, but