Page 245 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 245
P1: FJU/FFV P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN009A-426 July 6, 2001 20:44
448 Metal Hydrides
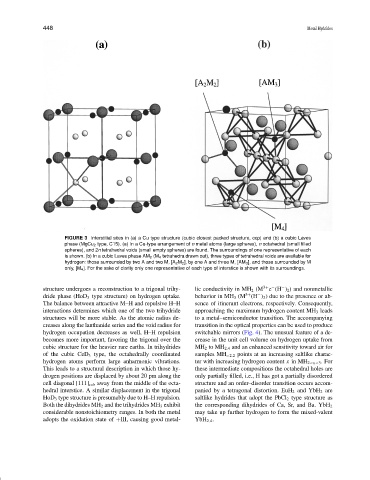
FIGURE 3 Interstitial sites in (a) a Cu type structure (cubic closest packed structure, ccp) and (b) a cubic Laves
phase (MgCu 2 type, C15). (a) In a Cu-type arrangement of n metal atoms (large spheres), n octahedral (small filled
spheres), and 2n tetrahedral voids (small empty spheres) are found. The surroundings of one representative of each
is shown. (b) In a cubic Laves phase AM 2 (M 4 tetrahedra drawn out), three types of tetrahedral voids are available for
hydrogen: those surrounded by two A and two M, [A 2 M 2 ], by one A and three M, [AM 3 ], and those surrounded by M
only, [M 4 ]. For the sake of clarity only one representative of each type of interstice is shown with its surroundings.
−
structure undergoes a reconstruction to a trigonal trihy- lic conductivity in MH 2 (M e (H ) 2 ) and nonmetallic
3+ −
3+
−
dride phase (HoD 3 type structure) on hydrogen uptake. behavior in MH 3 (M (H ) 3 ) due to the presence or ab-
The balance between attractive M–H and repulsive H–H sence of itinerant electrons, respectively. Consequently,
interactions determines which one of the two trihydride approaching the maximum hydrogen content MH 3 leads
structures will be more stable. As the atomic radius de- to a metal–semiconductor transition. The accompanying
creases along the lanthanide series and the void radius for transition in the optical properties can be used to produce
hydrogen occupation decreases as well, H–H repulsion switchable mirrors (Fig. 4). The unusual feature of a de-
becomes more important, favoring the trigonal over the crease in the unit cell volume on hydrogen uptake from
cubic structure for the heavier rare earths. In trihydrides MH 2 to MH 2.4 and an enhanced sensitivity toward air for
of the cubic CeD 3 type, the octahedrally coordinated samples MH >2.2 points at an increasing saltlike charac-
hydrogen atoms perform large anharmonic vibrations. ter with increasing hydrogen content x in MH 2<x<3 .For
This leads to a structural description in which those hy- these intermediate compositions the octahedral holes are
drogen positions are displaced by about 20 pm along the only partially filled, i.e., H has got a partially disordered
cell diagonal [111] cub away from the middle of the octa- structure and an order–disorder transition occurs accom-
hedral interstice. A similar displacement in the trigonal panied by a tetragonal distortion. EuH 2 and YbH 2 are
HoD 3 type structure is presumably due to H–H repulsion. saltlike hydrides that adopt the PbCl 2 type structure as
Both the dihydrides MH 2 and the trihydrides MH 3 exhibit the corresponding dihydrides of Ca, Sr, and Ba. YbH 2
considerable nonstoichiometry ranges. In both the metal may take up further hydrogen to form the mixed-valent
adopts the oxidation state of +III, causing good metal- YbH 2.4 .