Page 247 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 247
P1: FJU/FFV P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN009A-426 July 6, 2001 20:44
450 Metal Hydrides
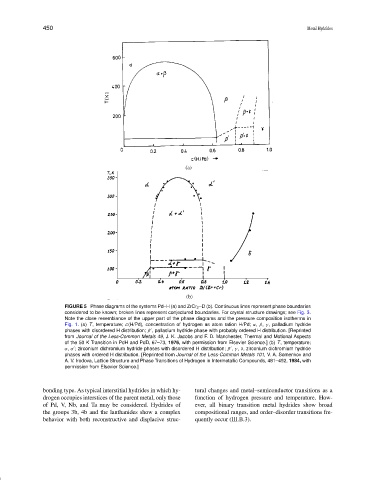
FIGURE 5 Phase diagrams of the systems Pd–H (a) and ZrCr 2 –D (b). Continuous lines represent phase boundaries
considered to be known; broken lines represent conjectured boundaries. For crystal structure drawings; see Fig. 3.
Note the close resemblance of the upper part of the phase diagrams and the pressure composition isotherms in
Fig. 1. (a) T, temperature; c(H/Pd), concentration of hydrogen as atom ration H/Pd; α, β, γ , palladium hydride
phases with disordered H distribution; β , palladium hydride phase with probably ordered H distribution. [Reprinted
from Journal of the Less-Common Metals 49, J. K. Jacobs and F. D. Manchester, Thermal and Motional Aspects
of the 50 K Transition in PdH and PdD, 67–73, 1976, with permission from Elsevier Science.] (b) T, temperature;
α, α ; zirconium dichromium hydride phases with disordered H distribution; β , γ , δ, zirconium dichromium hydride
phases with ordered H distribution. [Reprinted from Journal of the Less-Common Metals 101, V. A. Somenkov and
A. V. Irodova, Lattice Structure and Phase Transitions of Hydrogen in Intermetallic Compounds, 481–492, 1984, with
permission from Elsevier Science.]
bonding type. As typical interstitial hydrides in which hy- tural changes and metal–semiconductor transitions as a
drogen occupies interstices of the parent metal, only those function of hydrogen pressure and temperature. How-
of Pd, V, Nb, and Ta may be considered. Hydrides of ever, all binary transition metal hydrides show broad
the groups 3b, 4b and the lanthanides show a complex compositional ranges, and order–disorder transitions fre-
behavior with both reconstructive and displacive struc- quently occur (III.B.3).