Page 252 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 252
P1: FJU/FFV P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN009A-426 July 6, 2001 20:44
Metal Hydrides 455
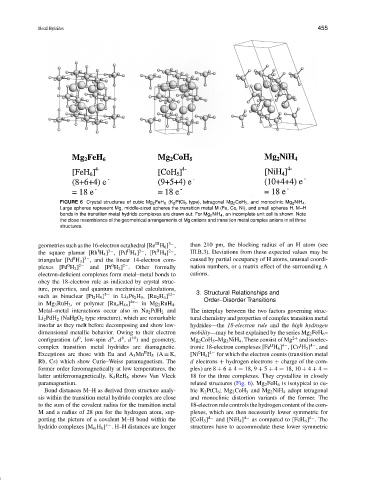
FIGURE 6 Crystal structures of cubic Mg 2 FeH 6 (K 2 PtCl 6 type), tetragonal Mg 2 CoH 5 , and monoclinic Mg 2 NiH 4 .
Large spheres represent Mg, middle-sized spheres the transition metal M (Fe, Co, Ni), and small spheres H. M–H
bonds in the transition metal hydrido complexes are drawn out. For Mg 2 NiH 4 , an incomplete unit cell is shown. Note
the close resemblance of the geometrical arrangements of Mg cations and transition metal complex anions in all three
structures.
III
geometries such as the 16-electron octahedral [Re H 6 ] , than 210 pm, the blocking radius of an H atom (see
3−
II
II
I
the square plamar [Rh H 4 ] , [Pd H 4 ] , [Pt H 4 ] , III.B.3). Deviations from these expected values may be
3−
2−
2−
0
triangular [Pd H 3 ] , and the linear 14-electron com- caused by partial occupancy of H atoms, unusual coordi-
3−
0
0
2−
plexes [Pd H 2 ] 2− and [Pt H 2 ] . Other formally nation numbers, or a matrix effect of the surrounding A
electron-deficient complexes form metal–metal bonds to cations.
obey the 18-electron rule as indicated by crystal struc-
ture, properties, and quantum mechanical calculations,
3. Structural Relationships and
such as binuclear [Pt 2 H 9 ] 5− in Li 5 Pt 2 H 9 , [Ru 2 H 6 ] 12−
Order–Disorder Transitions
in Mg 3 RuH 3 , or polymer [Ru n H 4n ] 4n− in Mg 2 RuH 4 .
Metal–metal interactions occur also in Na 2 PdH 2 and The interplay between the two factors governing struc-
Li 2 PdH 2 (NaHgO 2 type structure), which are remarkable tural chemistry and properties of complex transition metal
insofar as they melt before decomposing and show low- hydrides—the 18-electron rule and the high hydrogen
dimensional metallic behavior. Owing to their electron mobility—may be best explained by the series Mg 2 FeH 6 –
6
10
8
0
configuration (d , low-spin d , d , d ) and geometry, Mg 2 CoH 5 –Mg 2 NiH 4 . These consist of Mg 2+ and isoelec-
I
II
4−
4−
complex transition metal hydrides are diamagnetic. tronic 18-electron complexes [Fe H 6 ] , [Co H 5 ] , and
II
0
Exceptions are those with Eu and A 3 Mn H 5 (A = K, [Ni H 4 ] 4− for which the electron counts (transition metal
Rb, Cs) which show Curie–Weiss paramagnetism. The d electrons + hydrogen electrons + charge of the com-
former order ferromagnetically at low temperatures, the plex) are 8 + 6 + 4 = 18, 9 + 5 + 4 = 18, 10 + 4 + 4 =
latter antiferromagnetically. K 3 ReH 6 shows Van Vleck 18 for the three complexes. They crystallize in closely
paramagnetism. related structures (Fig. 6). Mg 2 FeH 6 is isotypical to cu-
Bond distances M–H as derived from structure analy- bic K 2 PtCl 6 ;Mg 2 CoH 5 and Mg 2 NiH 4 adopt tetragonal
sis within the transition metal hydrido complex are close and monoclinic distortion variants of the former. The
to the sum of the covalent radius for the transition metal 18-electron rule controls the hydrogen content of the com-
M and a radius of 28 pm for the hydrogen atom, sup- plexes, which are then necessarily lower symmetric for
4−
porting the picture of a covalent M–H bond within the [CoH 5 ] 4− and [NiH 4 ] 4− as compared to [FeH 6 ] . The
x−
hydrido complexes [M m H h ] .H–H distances are longer structures have to accommodate these lower symmetric