Page 357 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 357
P1: GTQ/GUU P2: GTQ Final Pages
Encyclopedia of Physical Science and Technology EN011H-551 July 25, 2001 18:33
676 Periodic Table (Chemistry)
D. Periodic Systems of Lothar Meyer Gustavus Detlef Henrichs. Odling’s system was based,
and Others in large measure, on the properties of compounds and
the recognition of trends in the formulas of analogous
Although Mendeleev is the major figure in the develop-
compounds. For example, he noted the pattern formed
ment of the periodic system of the elements, Julius Lothar
by the nonmetal hydrides: CH 4 ,NH 3 ,H 2 O, and HF.
Meyer proposed a very similar arrangement at about the
Henrichs was a European-born chemist who spent much
same time. Trained as a physician, Meyer ultimately be-
of his career as a professor of physical science at the
cameprofessorofchemistry and rectorof theUniversity of
University of Iowa. He also came up with an elemen-
T¨ubingen in southern Germany. Like Mendeleev, his mo-
tary classification which suggested a periodic variation of
tivation for organizing the elements was associated with
chemical and physical properties as a function of atomic
writing a textbook. Some of Meyer’s preliminary attempts
mass.
at a periodic classification date from 1864, but his first
real publication on the subject was in 1869. He stressed
the importance of elementary combining powers, or va- II. FEATURES OF THE MODERN
lence, in his tabulation and emphasized the periodicity of PERIODIC TABLE
certain physical properties. An example of the latter is
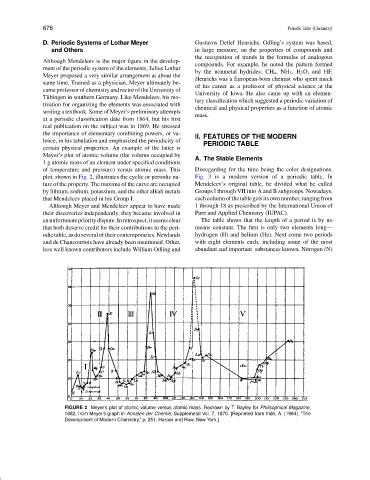
Meyer’s plot of atomic volume (the volume occupied by
A. The Stable Elements
1 g atomic mass of an element under specified conditions
of temperature and pressure) versus atomic mass. This Disregarding for the time being the color designations,
plot, shown in Fig. 2, illustrates the cyclic or periodic na- Fig. 3 is a modern version of a periodic table. In
ture of the property. The maxima of the curve are occupied Mendeleev’s original table, he divided what he called
by lithium, sodium, potassium, and the other alkali metals Groups I through VII into A and B subgroups. Nowadays,
that Mendeleev placed in his Group I. eachcolumnofthetablegetsitsownnumber,rangingfrom
Although Meyer and Mendeleev appear to have made 1 through 18 as prescribed by the International Union of
their discoveries independently, they became involved in Pure and Applied Chemistry (IUPAC).
anunfortunateprioritydispute.Inretrospect,itseemsclear The table shows that the length of a period is by no
that both deserve credit for their contributions to the peri- means constant. The first is only two elements long—
odic table, as do severalof their contemporaries. Newlands hydrogen (H) and helium (He). Next come two periods
and de Chancourtois have already been mentioned. Other, with eight elements each, including some of the most
less well known contributors include William Odling and abundant and important substances known. Nitrogen (N)
FIGURE 2 Meyer’s plot of atomic volume versus atomic mass. Redrawn by T. Bayley for Philisophical Magazine,
1882, from Meyer’s graph in Annalen der Chemie, Supplemetal Vol. 7, 1870. [Reprinted from Ihde, A. (1964). “The
Development of Modern Chemistry,” p. 251, Harper and Row, New York.]