Page 356 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 356
P1: GTQ/GUU P2: GTQ Final Pages
Encyclopedia of Physical Science and Technology EN011H-551 July 25, 2001 18:33
Periodic Table (Chemistry) 675
scientifically sound system. According to the historical TABLE I Predicted Properties of Ekasilicon and Observed
account, on February 17, 1869, Mendeleev sat at his desk, Properties of Germanium
arranging a stack of cards. Each carried the name, sym- Predicted Observed
bol, and atomic mass of a different element. In what has
been likened to a game of patience or solitaire, he laid Atomic mass 72 72.6
out the cards in rows in order of increasing atomic mass. Color of element 3 Gray Gray
Again, he saw what others had observed: when arranged in Density of element (g/cm ) 5.5 5.36
this fashion, elements naturally fall into families exhibit- Formula of oxide 3 XO 2 GeO 2
ing similar chemical properties which periodically repeat Density of oxide (g/cm ) 4.7 4.703
themselves. In fact, the properties of the elements appear Formula of chloride 3 XCl 4 GeCl 4
to be a function of the atomic mass, or in Mendeleev’s Density of chloride (g/cm ) 1.9 1.887
◦
words, “the size of the atomic weight determines the na- Boiling point of chloride ( C) <100 86
ture of the elements.” That conclusion appeared in a paper
on “The Relation of the Properties to the Atomic Weights
of the Elements,” read within a month of the February similar elements in the same vertical columns. They soon
discovery. would have been scattered all over the table. Titanium
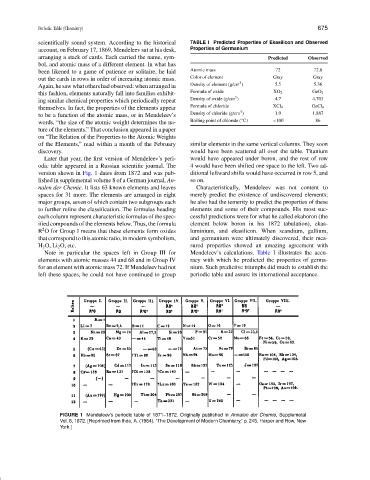
Later that year, the first version of Mendeleev’s peri- would have appeared under boron, and the rest of row
odic table appeared in a Russian scientific journal. The 4 would have been shifted one space to the left. Two ad-
version shown in Fig. 1 dates from 1872 and was pub- ditional leftward shifts would have occurred in row 5, and
lished in supplemental volume 8 of a German journal, An- so on.
nalen der Chemie. It lists 63 known elements and leaves Characteristically, Mendeleev was not content to
spaces for 31 more. The elements are arranged in eight merely predict the existence of undiscovered elements;
major groups, seven of which contain two subgroups each he also had the temerity to predict the properties of these
to further refine the classification. The formulas heading elements and some of their compounds. His most suc-
each column represent characteristic formulas of the spec- cessful predictions were for what he called ekaboron (the
ified compounds of the elements below. Thus, the formula element below boron in his 1872 tabulation), ekaa-
2
R O for Group I means that these elements form oxides luminium, and ekasilicon. When scandium, gallium,
that correspond to this atomic ratio, in modern symbolism, and germanium were ultimately discovered, their mea-
H 2 O, Li 2 O, etc. sured properties showed an amazing agreement with
Note in particular the spaces left in Group III for Mendeleev’s calculations. Table I illustrates the accu-
elements with atomic masses 44 and 68 and in Group IV racy with which he predicted the properties of germa-
for an element with atomic mass 72. If Mendeleev had not nium. Such predictive triumphs did much to establish the
left these spaces, he could not have continued to group periodic table and assure its international acceptance.
FIGURE 1 Mendeleev’s periodic table of 1871–1872. Originally published in Annalen der Chemie, Supplemetal
Vol. 8, 1872. [Reprinted from Ihde, A. (1964). “The Development of Modern Chemistry,” p. 245, Harper and Row, New
York.]