Page 381 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 381
P1: GQT/GRI P2: GTV/FFV P3: GTV/FFV QC: GSS Final pages
Encyclopedia of Physical Science and Technology EN014A-653 July 28, 2001 20:55
Rare Earth Elements and Materials 5
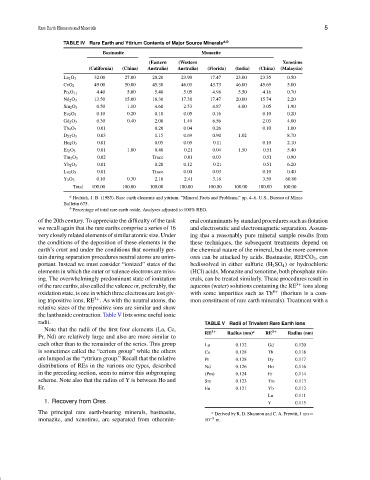
TABLE IV Rare Earth and Yttrium Contents of Major Source Minerals a,b
Bastnasite Monazite
(Eastern (Western Xenotime
(California) (China) Australia) Australia) (Florida) (India) (China) (Malaysia)
La 2 O 3 32.00 27.00 20.20 23.90 17.47 23.00 23.35 0.50
CeO 2 49.00 50.00 45.30 46.03 43.73 46.00 45.69 5.00
Pr 6 O 11 4.40 5.00 5.40 5.05 4.98 5.50 4.16 0.70
Nd 2 O 3 13.50 15.00 18.30 17.38 17.47 20.00 15.74 2.20
Sm 2 O 3 0.50 1.10 4.60 2.53 4.87 4.00 3.05 1.90
Eu 2 O 3 0.10 0.20 0.10 0.05 0.16 0.10 0.20
Gd 2 O 3 0.30 0.40 2.00 1.49 6.56 2.03 4.00
Tb 4 O 7 0.01 0.20 0.04 0.26 0.10 1.00
Dy 2 O 3 0.03 1.15 0.69 0.90 1.02 8.70
Ho 2 O 3 0.01 0.05 0.05 0.11 0.10 2.10
Er 2 O 3 0.01 1.00 0.40 0.21 0.04 1.50 0.51 5.40
Tm 2 O 3 0.02 Trace 0.01 0.03 0.51 0.90
Yb 2 O 3 0.01 0.20 0.12 0.21 0.51 6.20
Lu 2 O 3 0.01 Trace 0.04 0.03 0.10 0.40
Y 2 O 3 0.10 0.30 2.10 2.41 3.18 3.50 60.80
Total 100.00 100.00 100.00 100.00 100.00 100.00 100.00 100.00
a Hedrick, J. B. (1985). Rare earth elements and yttrium. “Mineral Facts and Problems,” pp. 4–6. U.S., Bureau of Mines
Bulletin 675.
b Percentage of total rare earth oxide. Analyses adjusted to 100% REO.
of the 20th century. To appreciate the difficulty of the task eral contaminants by standard procedures such as flotation
we recall again that the rare earths comprise a series of 16 and electrostatic and electromagnetic separation. Assum-
very closely related elements of similar atomic size. Under ing that a reasonably pure mineral sample results from
the conditions of the deposition of these elements in the these techniques, the subsequent treatments depend on
earth’s crust and under the conditions that normally per- the chemical nature of the mineral, but the more common
tain during separation procedures neutral atoms are unim- ores can be attacked by acids. Bastnasite, REFCO 3 , can
portant. Instead we must consider “ionized” states of the bedissolved in either sulfuric (H 2 SO 4 ) or hydrochloric
elements in which the outer or valence electrons are miss- (HCl) acids. Monazite and xenotime, both phosphate min-
ing. The overwhelmingly predominant state of ionization erals, can be treated similarly. These procedures result in
of the rare earths, also called the valence or, preferably, the aqueous (water) solutions containing the RE 3+ ions along
oxidation state, is one in which three electrons are lost giv- with some impurities such as Th 4+ (thorium is a com-
ing tripositive ions, RE . As with the neutral atoms, the mon constituent of rare earth minerals). Treatment with a
3+
relative sizes of the tripositive ions are similar and show
the lanthanide contraction. Table V lists some useful ionic
radii. TABLE V Radii of Trivalent Rare Earth Ions
Note that the radii of the first four elements (La, Ce,
RE 3+ Radius (nm) a RE 3+ Radius (nm)
Pr, Nd) are relatively large and also are more similar to
each other than to the remainder of the series. This group La 0.132 Gd 0.120
is sometimes called the “cerium group” while the others Ce 0.128 Tb 0.118
are lumped as the “yttrium group.” Recall that the relative Pr 0.128 Dy 0.117
distributions of REs in the various ore types, described Nd 0.126 Ho 0.116
in the preceding section, seem to mirror this subgrouping (Pm) 0.124 Er 0.114
scheme. Note also that the radius of Y is between Ho and Sm 0.123 Tm 0.113
Er. Eu 0.121 Yb 0.112
Lu 0.111
1. Recovery from Ores Y 0.115
The principal rare earth-bearing minerals, bastnasite, a
Derived by R. D. Shannon and C. A. Prewitt, 1 nm =
monazite, and xenotime, are separated from othermin- 10 −9 m.