Page 385 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 385
P1: GQT/GRI P2: GTV/FFV P3: GTV/FFV QC: GSS Final pages
Encyclopedia of Physical Science and Technology EN014A-653 July 28, 2001 20:55
Rare Earth Elements and Materials 9
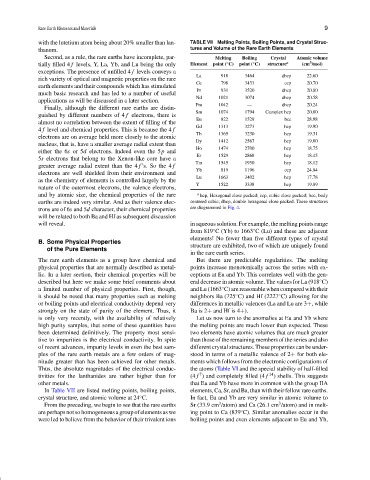
with the lutetium atom being about 20% smaller than lan- TABLE VII Melting Points, Boiling Points, and Crystal Struc-
thanum. tures and Volume of the Rare Earth Elements
Second, as a rule, the rare earths have incomplete, par- Melting Boiling Crystal Atomic volume
3
tially filled 4 f levels, Y, La, Yb, and Lu being the only Element point ( C) point ( C) structure a (cm /mol)
◦
◦
exceptions. The presence of unfilled 4 f levels conveys a
La 918 3464 dhcp 22.60
rich variety of optical and magnetic properties on the rare
Ce 798 3433 ccp 20.70
earth elements and their compounds which has stimulated
Pr 931 3520 dhcp 20.80
much basic research and has led to a number of useful
Nd 1021 3074 dhcp 20.58
applications as will be discussed in a later section.
Pm 1042 — dhcp 20.24
Finally, although the different rare earths are distin-
Sm 1074 1794 Complex hcp 20.00
guished by different numbers of 4 f electrons, there is
Eu 822 1529 bcc 28.98
almost no correlation between the extent of filling of the
Gd 1313 3273 hcp 19.90
4 f level and chemical properties. This is because the 4 f
Tb 1365 3230 hcp 19.31
electrons are on average held more closely to the atomic
Dy 1412 2567 hcp 19.00
nucleus, that is, have a smaller average radial extent than
Ho 1474 2700 hcp 18.75
either the 6s or 5d electrons. Indeed even the 5p and
Er 1529 2868 hcp 18.45
5s electrons that belong to the Xenon-like core have a
Tm 1545 1950 hcp 18.12
greater average radial extent than the 4 f ’s. So the 4 f
Yb 819 1196 ccp 24.84
electrons are well shielded from their environment and
Lu 1663 3402 hcp 17.78
as the chemistry of elements is controlled largely by the
Y 1522 3338 hcp 19.89
nature of the outermost electrons, the valence electrons,
and by atomic size, the chemical properties of the rare a hcp, Hexagonal close packed; ccp, cubic close packed; bcc, body
earths are indeed very similar. And as their valence elec- centered cubic; dhcp, double hexagonal close packed. These structures
trons are of 6s and 5d character, their chemical properties are diagrammed in Fig. 4.
will be related to both Ba and Hf as subsequent discussion
will reveal. in aqueous solution. For example, the melting points range
from 819 C (Yb) to 1663 C (Lu) and these are adjacent
◦
◦
elements! No fewer than five different types of crystal
B. Some Physical Properties
structure are exhibited, two of which are uniquely found
of the Pure Elements
in the rare earth series.
The rare earth elements as a group have chemical and But there are predictable regularities. The melting
physical properties that are normally described as metal- points increase monotonically across the series with ex-
lic. In a later section, their chemical properties will be ceptions at Eu and Yb. This correlates well with the gen-
described but here we make some brief comments about eral decrease in atomic volume. The values for La (918 C)
◦
◦
a limited number of physical properties. First, though, and Lu (1663 C) are reasonable when compared with their
◦
it should be noted that many properties such as melting neighbors Ba (725 C) and Hf (2227 C) allowing for the
◦
or boiling points and electrical conductivity depend very differences in metallic valences (La and Lu are 3+, while
strongly on the state of purity of the element. Thus, it Ba is 2+ and Hf is 4+).
is only very recently, with the availability of relatively Let us now turn to the anomalies at Eu and Yb where
high purity samples, that some of these quantities have the melting points are much lower than expected. These
been determined definitively. The property most sensi- two elements have atomic volumes that are much greater
tive to impurities is the electrical conductivity. In spite than those of the remaining members of the series and also
of recent advances, impurity levels in even the best sam- different crystal structures. These properties can be under-
ples of the rare earth metals are a few orders of mag- stood in terms of a metallic valence of 2+ for both ele-
nitude greater than has been achieved for other metals. ments which follows from the electronic configurations of
Thus, the absolute magnitudes of the electrical conduc- the atoms (Table VI and the special stability of half-filled
14
7
tivities for the lanthanides are rather higher than for (4 f ) and completely filled (4 f ) shells. This suggests
other metals. that Eu and Yb have more in common with the group IIA
In Table VII are listed melting points, boiling points, elements, Ca, Sr, and Ba, than with their fellow rare earths.
crystal structure, and atomic volume at 24 C. In fact, Eu and Yb are very similar in atomic volume to
◦
3
3
From the preceding, we begin to see that the rare earths Sr (33.9 cm /atom) and Ca (26.1 cm /atom) and in melt-
◦
are perhaps not so homogeneous a group of elements as we ing point to Ca (839 C). Similar anomalies occur in the
were led to believe from the behavior of their trivalent ions boiling points and even elements adjacent to Eu and Yb,