Page 389 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 389
P1: GQT/GRI P2: GTV/FFV P3: GTV/FFV QC: GSS Final pages
Encyclopedia of Physical Science and Technology EN014A-653 July 28, 2001 20:55
Rare Earth Elements and Materials 13
TABLE VIII Magnetic Properties of the Rare finalproductofthecalcinationinairofmostREmetalsand
Earth Metals salts. This is a consequence of the high thermodynamic
Metal T c (K) Type of order a affinity of the rare earth elements for oxygen and the sta-
bility of their trivalent oxidation state. The three elements:
Ce 12.5 Af(?) cerium, praseodymium, and terbium, however, have stable
Pr — No order oxides of different compositions, namely, CeO 2 , Pr 6 O 11 ,
Nd 19.2 AF and Tb 4 O 7 , respectively. Cerium oxide, CeO 2 (also known
7.8 AF asceria)containsonlytetravalentCe,andisthemoststable
Pm — — form produced when most cerium salts, Ce 3+ or Ce , are
4+
Sm 106 F
calcinated in air. For Pr and Tb, stable oxides with mixed
13.8 Complex AF
+3/+4 composition result due to sufficient but limited
Eu 91 AF
stability of the tetravalent state. The coexistence of two
Gd 293 F
valence state in the solid makes charge-transfer absorption
Tb 230 AF spiral
bands possible and these two oxides are strongly colored.
225 F
Mixed valence oxides RE 3 O 4 (RE = for example, Eu or
Dy 146 AF spiral 2+ 3+ 2−
Sm), which can be formulated as RE (RE ) 2 (O ) 4 ,
90 F
also exist.
133 AF spiral
Divalent oxides REO (RE = Nd, Sm, Eu, Yb) exist and
42 Complex
may be prepared by reduction of RE 2 O 3 with elemental
20 F-Cone spiral
rare earths at high temperature and pressure:
Er 80 Complex sine wave
52 Complex Ln 2 O 3 + Ln ⇒ 3LnO
20 F-Cone NdO, SmO exhibit lustrous golden yellow colors, ex-
Tm 56 Complex hibit metallic conductivity, and may be formulated as
32 Complex RE (O )(e ), whereas EuO (dark red), YbO (greyish-
2−
−
3+
2−
2+
a AF, antiferromagnetic; F, ferromagnetic. white) are insulating genuine RE O . Such differences
may be understood in terms of their electron configuration.
n
1
It appears that the [Xe]4 f 5d configuration is more sta-
ble than the [Xe] f n+1 in some rare earth compounds, con-
tributing a single electron per rare earth atom to a broad 5d
band. The partially filled 5d band is believed to be respon-
sible for the high electrical conductivity of NdO and SmO.
The rare earths also form a large number of “complex”
oxides that involve one or more other metals in addition
to a rare earth and oxygen. The most numerous and im-
portant type are oxides of composition REFeO 3 , RECrO 3 ,
and similar types called “perovskites” and those of com-
position RE 3 Fe 5 O 12 or RE 3 Al 5 O 12 called “garnets.” The
magneticcharacteristicsofrareearthgarnetshavebeenex-
ploited for “magnetic bubble” memory in computer stor-
age system. The best bubble materials are 20-µm films of
RE 3 Fe 5 O 12 .
2. Hydrides
All of the rare earths combine directly with hydrogen to
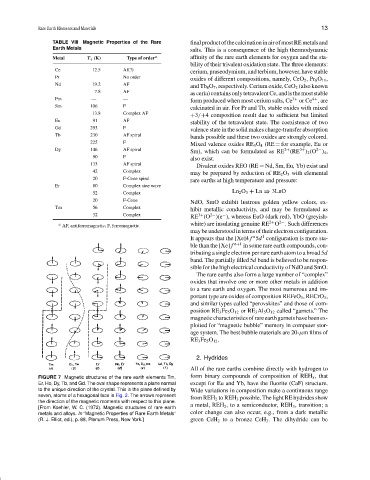
FIGURE 7 Magnetic structures of the rare earth elements Tm, form binary compounds of composition of REH x , that
Er, Ho, Dy, Tb, and Gd. The oval shape represents a plane normal except for Eu and Yb, have the fluorite (CaF) structure.
to the unique direction of the crystal. This is the plane defined by Wide variations in composition make a continuous range
seven, atoms of a hexagonal face in Fig. 2. The arrows represent from REH 2 to REH 3 possible. The light RE hydrides show
the direction of the magnetic moments with respect to this plane.
[From Koehler, W. C. (1972). Magnetic structures of rare earth a metal, REH 2 , to a semiconductor, REH 3 , transition; a
metals and alloys. In “Magnetic Properties of Rare Earth Metals” color change can also occur, e.g., from a dark metallic
(R. J. Elliot, ed.), p. 88, Plenum Press, New York.] green CeH 2 to a bronze CeH 3 . The dihydride can be