Page 66 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 66
P1: LLL Revised Pages
Encyclopedia of Physical Science and Technology EN002c-73 May 21, 2001 13:59
Boron Hydrides 315
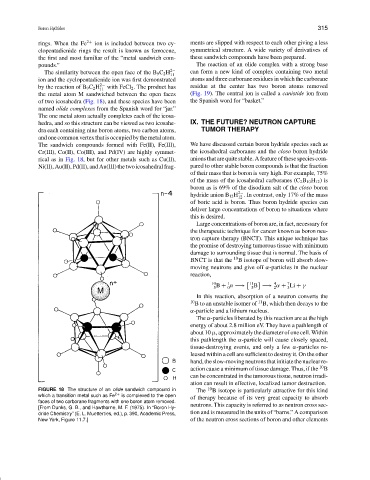
rings. When the Fe 2+ ion is included between two cy- ments are slipped with respect to each other giving a less
clopentadienide rings the result is known as ferrocene, symmetrical structure. A wide variety of derivatives of
the first and most familiar of the “metal sandwich com- these sandwich compounds have been prepared.
pounds.” The reaction of an olide complex with a strong base
The similarity between the open face of the B 9 C 2 H 2− can form a new kind of complex containing two metal
11
ion and the cyclopentadienide ion was first demonstrated atoms and three carborane residues in which the carborane
by the reaction of B 9 C 2 H 2− with FeCl 2 . The product has residue at the center has two boron atoms removed
11
the metal atom M sandwiched between the open faces (Fig. 19). The central ion is called a canistide ion from
of two icosahedra (Fig. 18), and these species have been the Spanish word for “basket.”
named olide complexes from the Spanish word for “jar.”
The one metal atom actually completes each of the icosa-
hedra, and so this structure can be viewed as two icosahe- IX. THE FUTURE? NEUTRON CAPTURE
dra each containing nine boron atoms, two carbon atoms, TUMOR THERAPY
andonecommonvertexthatisoccupiedbythemetalatom.
The sandwich compounds formed with Fe(II), Fe(III), We have discussed certain boron hydride species such as
Cr(III), Co(II), Co(III), and Pd(IV) are highly symmet- the icosahedral carboranes and the closo boron hydride
rical as in Fig. 18, but for other metals such as Cu(II), anions that are quite stable. A feature of these species com-
Ni(II), Au(II), Pd(II), and Au(III) the two icosahedral frag- pared to other stable boron compounds is that the fraction
of their mass that is boron is very high. For example, 75%
of the mass of the icosahedral carboranes (C 2 B 10 H 12 )is
boron as is 69% of the disodium salt of the closo boron
2−
hydride anion B 12 H . In contrast, only 17% of the mass
12
of boric acid is boron. Thus boron hydride species can
deliver large concentrations of boron to situations where
this is desired.
Large concentrations of boron are, in fact, necessary for
the therapeutic technique for cancer known as boron neu-
tron capture therapy (BNCT). This unique technique has
the promise of destroying tumorous tissue with minimum
damage to surrounding tissue that is normal. The basis of
BNCT is that the 10 B isotope of boron will absorb slow-
moving neutrons and give off α-particles in the nuclear
reaction,
10 1 11 4 7
3
2
0
5 B + n −→ 5 B −→ α + Li + γ
In this reaction, absorption of a neutron converts the
10 11
B to an unstable isomer of B, which then decays to the
α-particle and a lithium nucleus.
The α-particles liberated by this reaction are at the high
energy of about 2.8 million eV. They have a pathlength of
about 10 µ, approximately the diameter of one cell. Within
this pathlength the α-particle will cause closely spaced,
tissue-destroying events, and only a few α-particles re-
leased within a cell are sufficient to destroy it. On the other
hand,theslow-movingneutronsthatinitiatethenuclearre-
10
action cause a minimum of tissue damage. Thus, if the B
can be concentrated in the tumorous tissue, neutron irradi-
ation can result in effective, localized tumor destruction.
FIGURE 18 The structure of an olide sandwich compound in The 10 B isotope is particularly attractive for this kind
which a transition metal such as Fe 2+ is complexed to the open of therapy because of its very great capacity to absorb
faces of two carborane fragments with one boron atom removed.
[From Dunks, G. B., and Hawthorne, M. F. (1975). In “Boron Hy- neutrons. This capacity is referred to as neutron cross sec-
dride Chemistry” (E. L. Muetterties, ed.), p. 390, Academic Press, tion and is measured in the units of “barns.” A comparison
New York, Figure 11.7.] of the neutron cross sections of boron and other elements