Page 64 - Academic Press Encyclopedia of Physical Science and Technology 3rd Molecular Biology
P. 64
P1: GRA Final
Encyclopedia of Physical Science and Technology EN006H-655 June 29, 2001 21:21
Gene Expression, Regulation of 513
RNA splice site choice. The X chromosome encodes for
transcription factors that control Sex-lethal (Sxl) transcrip-
tion. In females, which contain two X chromosomes, the
double dose of these transcription factors results in an acti-
vation of an early promoter of the Sxl gene. This promoter
is inactive in males, which contain one X chromosome.
The female-specific Sxl protein is an RNA-binding pro-
tein that binds to certain pyrimidine tracts and outcom-
petes U2AF binding to that site. Since the Sxl protein
lacks the splicing activator function of U2AF, Sxl binding
to a pyrimidine tract prohibits spliceosome formation at
the 3 splice site. Thus, once made, the female-specific
Sxl protein autoregulates its own expression by ensuring
that exon 3 in the Sxl pre-mRNA is efficiently skipped,
thereby establishing a female-specific splicing of the Sxl
pre-mRNA (Fig. 11). In male flies exon 3 is incorporated
during splicing, resulting in the translation of a function-
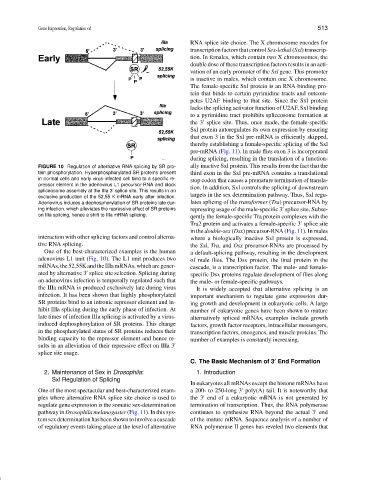
FIGURE 10 Regulation of alternative RNA splicing by SR pro- ally inactive Sxl protein. This results from the fact that the
tein phosphorylation. Hyperphosphorylated SR proteins present third exon in the Sxl pre-mRNA contains a translational
in normal cells and early virus-infected cell bind to a specific re-
stop codon that causes a premature termination of transla-
pressor element in the adenovirus L1 precursor-RNA and block
tion. In addition, Sxl controls the splicing of downstream
spliceosome assembly at the IIIa 3 splice site. This results in an
exclusive production of the 52,55 K mRNA early after infection. targets in the sex determination pathway. Thus, Sxl regu-
Adenovirus induces a dephosphorylation of SR proteins late dur- lates splicing of the transformer (Tra) precursor-RNA by
ing infection, which alleviates the repressive effect of SR proteins repressing usage of the male-specific 3 splice site. Subse-
on IIIa splicing, hence a shift to IIIa mRNA splicing. qently the female-specific Tra protein complexes with the
Tra2 protein and activates a female-specific 3 splice site
in the double-sex (Dsx) precursor-RNA (Fig. 11). In males
interaction with other splicing factors and control alterna- where a biologically inactive Sxl protein is expressed,
tive RNA splicing. the Sxl, Tra, and Dsx precursor-RNAs are processed by
One of the best-characterized examples is the human a default-splicing pathway, resulting in the development
adenovirus L1 unit (Fig. 10). The L1 unit produces two of male flies. The Dsx protein, the final protein in the
mRNAs,the52,55KandtheIIIamRNAs,whicharegener- cascade, is a transcription factor. The male- and female-
ated by alternative 3 splice site selection. Splicing during specific Dsx proteins regulate development of flies along
an adenovirus infection is temporally regulated such that the male- or female-specific pathways.
the IIIa mRNA is produced exclusively late during virus It is widely accepted that alternative splicing is an
infection. It has been shown that highly phosphorylated important mechanism to regulate gene expression dur-
SR proteins bind to an intronic repressor element and in- ing growth and development in eukaryotic cells. A large
hibit IIIa splicing during the early phase of infection. At number of eukaryotic genes have been shown to mature
late times of infection IIIa splicing is activated by a virus- alternatively spliced mRNAs, examples include growth
induced dephosphorylation of SR proteins. This change factors, growth factor receptors, intracellular messengers,
in the phosphorylated status of SR proteins reduces their transcription factors, oncogenes, and muscle proteins. The
binding capacity to the repressor element and hence re- number of examples is constantly increasing.
sults in an alleviation of their repressive effect on IIIa 3
splice site usage.
C. The Basic Mechanism of 3 End Formation
2. Maintenance of Sex in Drosophila: 1. Introduction
Sxl Regulation of Splicing
In eukaryotes all mRNAs except the histone mRNAs have
One of the most spectacular and best-characterized exam- a 200- to 250-long 3 poly(A) tail. It is noteworthy that
ples where alternative RNA splice site choice is used to the 3 end of a eukaryotic mRNA is not generated by
regulate gene expression is the somatic sex-determination termination of transcription. Thus, the RNA polymerase
pathway in Drosophila melanogaster (Fig. 11). In this sys- continues to synthesize RNA beyond the actual 3 end
tem sex determination has been shown to involve a cascade of the mature mRNA. Sequence analysis of a number of
of regulatory events taking place at the level of alternative RNA polymerase II genes has reveled two elements that