Page 71 - Subyek Encyclopedia - Encyclopedia of Separation Science
P. 71
66 I / CRYSTALLIZATION/ Derivatization
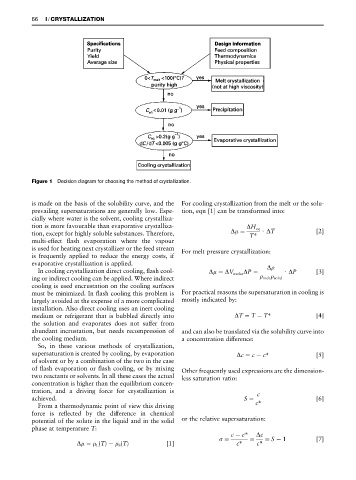
Figure 1 Decision diagram for choosing the method of crystallization.
is made on the basis of the solubility curve, and the For cooling crystallization from the melt or the solu-
prevailing supersaturations are generally low. Espe- tion, eqn [1] can be transformed into:
cially where water is the solvent, cooling crystalliza-
tion is more favourable than evaporative crystalliza- " H eq
tion, except for highly soluble substances. Therefore, TH ) T [2]
multi-effect Sash evaporation where the vapour
is used for heating next crystallizer or the feed stream For melt pressure crystallization:
is frequently applied to reduce the energy costs, if
evaporative crystallization is applied.
In cooling crystallization direct cooling, Sash cool- " V molar P" ) P [3]
ing or indirect cooling can be applied. Where indirect melt solid
cooling is used encrustation on the cooling surfaces
must be minimized. In Sash cooling this problem is For practical reasons the supersaturation in cooling is
largely avoided at the expense of a more complicated mostly indicated by:
installation. Also direct cooling uses an inert cooling
medium or refrigerant that is bubbled directly into T"T!TH [4]
the solution and evaporates does not suffer from
abundant incrustation, but needs recompression of and can also be translated via the solubility curve into
the cooling medium. a concentration difference:
So, in these various methods of crystallization,
supersaturation is created by cooling, by evaporation c"c!cH [5]
of solvent or by a combination of the two in the case
of Sash evaporation or Sash cooling, or by mixing Other frequently used expressions are the dimension-
two reactants or solvents. In all these cases the actual less saturation ratio:
concentration is higher than the equilibrium concen-
tration, and a driving force for crystallization is c
achieved. S" cH [6]
From a thermodynamic point of view this driving
force is reSected by the difference in chemical
potential of the solute in the liquid and in the solid or the relative supersaturation:
phase at temperature T:
" c!cH " c "S!1 [7]
" L (T)! S (T) [1] cH cH