Page 260 - Engineering Plastics Handbook
P. 260
222 Engineering Plastics
carbon fiber filler. It is in a crystalline state during injection molding,
™
because of the enhancement of the crystallization rate. SuperAurum has
better chemical and mechanical properties. The polymer was completed
through an optimum monomer selection.
Polymers—Chemistry of
Thermoplastic Polyimide[1]
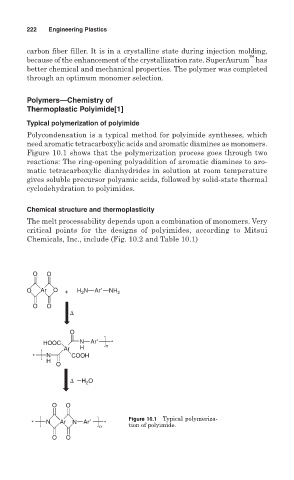
Typical polymerization of polyimide
Polycondensation is a typical method for polyimide syntheses, which
need aromatic tetracarboxylic acids and aromatic diamines as monomers.
Figure 10.1 shows that the polymerization process goes through two
reactions: The ring-opening polyaddition of aromatic diamines to aro-
matic tetracarboxylic dianhydrides in solution at room temperature
gives soluble precursor polyamic acids, followed by solid-state thermal
cyclodehydration to polyimides.
Chemical structure and thermoplasticity
The melt processability depends upon a combination of monomers. Very
critical points for the designs of polyimides, according to Mitsui
Chemicals, Inc., include (Fig. 10.2 and Table 10.1)
O O
O Ar O + H N Ar′ NH 2
2
O O
∆
O
HOOC N Ar′ n *
Ar H
* N COOH
H
O
∆ H O
2
O O
* N Ar N Ar′ * Figure 10.1 Typical polymeriza-
n tion of polyimide.
O O