Page 436 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 436
404 Enhanced Oil Recovery in Shale and Tight Reservoirs
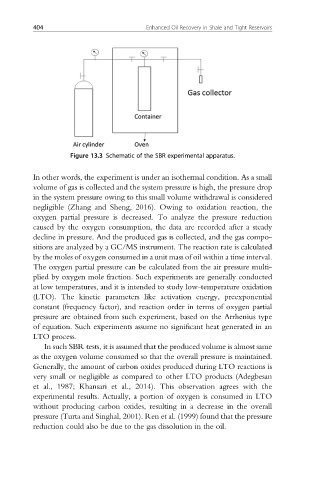
Figure 13.3 Schematic of the SBR experimental apparatus.
In other words, the experiment is under an isothermal condition. As a small
volume of gas is collected and the system pressure is high, the pressure drop
in the system pressure owing to this small volume withdrawal is considered
negligible (Zhang and Sheng, 2016). Owing to oxidation reaction, the
oxygen partial pressure is decreased. To analyze the pressure reduction
caused by the oxygen consumption, the data are recorded after a steady
decline in pressure. And the produced gas is collected, and the gas compo-
sitions are analyzed by a GC/MS instrument. The reaction rate is calculated
by the moles of oxygen consumed in a unit mass of oil within a time interval.
The oxygen partial pressure can be calculated from the air pressure multi-
plied by oxygen mole fraction. Such experiments are generally conducted
at low temperatures, and it is intended to study low-temperature oxidation
(LTO). The kinetic parameters like activation energy, preexponential
constant (frequency factor), and reaction order in terms of oxygen partial
pressure are obtained from such experiment, based on the Arrhenius type
of equation. Such experiments assume no significant heat generated in an
LTO process.
In such SBR tests, it is assumed that the produced volume is almost same
as the oxygen volume consumed so that the overall pressure is maintained.
Generally, the amount of carbon oxides produced during LTO reactions is
very small or negligible as compared to other LTO products (Adegbesan
et al., 1987; Khansari et al., 2014). This observation agrees with the
experimental results. Actually, a portion of oxygen is consumed in LTO
without producing carbon oxides, resulting in a decrease in the overall
pressure (Turta and Singhal, 2001). Ren et al. (1999) found that the pressure
reduction could also be due to the gas dissolution in the oil.