Page 494 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 494
458 Enhanced Oil Recovery in Shale and Tight Reservoirs
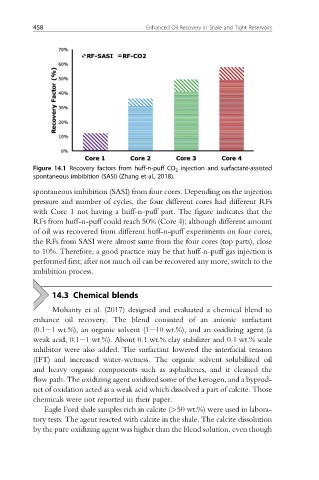
Figure 14.1 Recovery factors from huff-n-puff CO 2 injection and surfactant-assisted
spontaneous imbibition (SASI) (Zhang et al., 2018).
spontaneous imbibition (SASI) from four cores. Depending on the injection
pressure and number of cycles, the four different cores had different RFs
with Core 1 not having a huff-n-puff part. The figure indicates that the
RFs from huff-n-puff could reach 50% (Core 4); although different amount
of oil was recovered from different huff-n-puff experiments on four cores,
the RFs from SASI were almost same from the four cores (top parts), close
to 10%. Therefore, a good practice may be that huff-n-puff gas injection is
performed first; after not much oil can be recovered any more, switch to the
imbibition process.
14.3 Chemical blends
Mohanty et al. (2017) designed and evaluated a chemical blend to
enhance oil recovery. The blend consisted of an anionic surfactant
(0.1e1 wt.%), an organic solvent (1e10 wt.%), and an oxidizing agent (a
weak acid, 0.1e1 wt.%). About 0.1 wt.% clay stabilizer and 0.1 wt.% scale
inhibitor were also added. The surfactant lowered the interfacial tension
(IFT) and increased water-wetness. The organic solvent solubilized oil
and heavy organic components such as asphaltenes, and it cleaned the
flow path. The oxidizing agent oxidized some of the kerogen, and a byprod-
uct of oxidation acted as a weak acid which dissolved a part of calcite. Those
chemicals were not reported in their paper.
Eagle Ford shale samples rich in calcite (>50 wt.%) were used in labora-
tory tests. The agent reacted with calcite in the shale. The calcite dissolution
by the pure oxidizing agent was higher than the blend solution, even though