Page 93 - Environmental Control in Petroleum Engineering
P. 93
80 Environmental Control in Petroleum Engineering
the carbon chain can branch, as seen by the two isomers of butane,
the carbon chain does not form continuous loops.
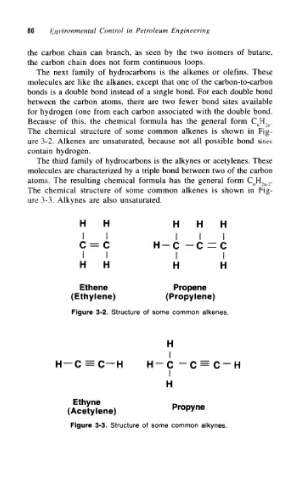
The next family of hydrocarbons is the alkenes or olefins. These
molecules are like the alkanes, except that one of the carbon-to-carbon
bonds is a double bond instead of a single bond. For each double bond
between the carbon atoms, there are two fewer bond sites available
for hydrogen (one from each carbon associated with the double bond.
Because of this, the chemical formula has the general form C nH, n.
The chemical structure of some common alkenes is shown in Fig-
ure 3-2. Alkenes are unsaturated, because not all possible bond sites
contain hydrogen.
The third family of hydrocarbons is the alkynes or acetylenes. These
molecules are characterized by a triple bond between two of the carbon
atoms. The resulting chemical formula has the general form C nH 2n-2.
The chemical structure of some common alkenes is shown in Fig-
ure 3-3. Alkynes are also unsaturated.
H H H H H
1 1 1 1 1
C = C H-C — C = C
1 1 1 1
H H H H
Ethene Propene
(Ethylene) (Propylene)
Figure 3-2. Structure of some common alkenes.
H
__ ! _
==
H C = C H H C C C H
1
H
Ethyne n
(Acetylene) Propyne
Figure 3-3. Structure of some common alkynes.