Page 109 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 109
Nanomaterials Fabrication 95
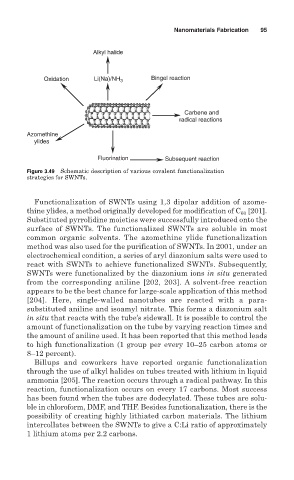
Alkyl halide
Oxidation Li(Na)/NH 3 Bingel reaction
Carbene and
radical reactions
Azomethine
ylides
Fluorination Subsequent reaction
Figure 3.49 Schematic description of various covalent functionalization
strategies for SWNTs.
Functionalization of SWNTs using 1,3 dipolar addition of azome-
thine ylides, a method originally developed for modification of C [201].
60
Substituted pyrrolidine moieties were successfully introduced onto the
surface of SWNTs. The functionalized SWNTs are soluble in most
common organic solvents. The azomethine ylide functionalization
method was also used for the purification of SWNTs. In 2001, under an
electrochemical condition, a series of aryl diazonium salts were used to
react with SWNTs to achieve functionalized SWNTs. Subsequently,
SWNTs were functionalized by the diazonium ions in situ generated
from the corresponding aniline [202, 203]. A solvent-free reaction
appears to be the best chance for large-scale application of this method
[204]. Here, single-walled nanotubes are reacted with a para-
substituted aniline and isoamyl nitrate. This forms a diazonium salt
in situ that reacts with the tube’s sidewall. It is possible to control the
amount of functionalization on the tube by varying reaction times and
the amount of aniline used. It has been reported that this method leads
to high functionalization (1 group per every 10–25 carbon atoms or
8–12 percent).
Billups and coworkers have reported organic functionalization
through the use of alkyl halides on tubes treated with lithium in liquid
ammonia [205]. The reaction occurs through a radical pathway. In this
reaction, functionalization occurs on every 17 carbons. Most success
has been found when the tubes are dodecylated. These tubes are solu-
ble in chloroform, DMF, and THF. Besides functionalization, there is the
possibility of creating highly lithiated carbon materials. The lithium
intercollates between the SWNTs to give a C:Li ratio of approximately
1 lithium atoms per 2.2 carbons.