Page 404 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 404
Nanomaterials as Adsorbents 387
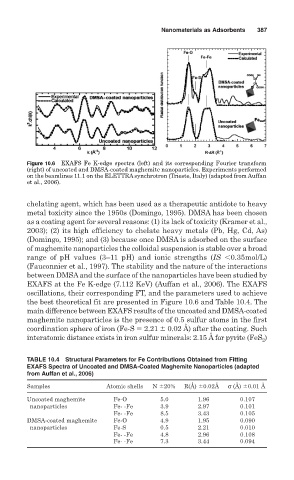
Figure 10.6 EXAFS Fe K-edge spectra (left) and its corresponding Fourier transform
(right) of uncoated and DMSA-coated maghemite nanoparticles. Experiments performed
on the beamlines 11.1 on the ELETTRA synchrotron (Trieste, Italy) (adapted from Auffan
et al., 2006).
chelating agent, which has been used as a therapeutic antidote to heavy
metal toxicity since the 1950s (Domingo, 1995). DMSA has been chosen
as a coating agent for several reasons: (1) its lack of toxicity (Kramer et al.,
2003); (2) its high efficiency to chelate heavy metals (Pb, Hg, Cd, As)
(Domingo, 1995); and (3) because once DMSA is adsorbed on the surface
of maghemite nanoparticles the colloidal suspension is stable over a broad
range of pH values (3–11 pH) and ionic strengths (IS 0.35mol/L)
(Fauconnier et al., 1997). The stability and the nature of the interactions
between DMSAand the surface of the nanoparticles have been studied by
EXAFS at the Fe K-edge (7.112 KeV) (Auffan et al., 2006). The EXAFS
oscillations, their corresponding FT, and the parameters used to achieve
the best theoretical fit are presented in Figure 10.6 and Table 10.4. The
main difference between EXAFS results of the uncoated and DMSA-coated
maghemite nanoparticles is the presence of 0.5 sulfur atoms in the first
coordination sphere of iron (Fe-S 2.21 ! 0.02 Å) after the coating. Such
)
interatomic distance exists in iron sulfur minerals: 2.15 Å for pyrite (FeS 2
TABLE 10.4 Structural Parameters for Fe Contributions Obtained from Fitting
EXAFS Spectra of Uncoated and DMSA-Coated Maghemite Nanoparticles (adapted
from Auffan et al., 2006)
Samples Atomic shells N !20% R(Å) !0.02Å σ (Å) !0.01 Å
Uncoated maghemite Fe-O 5.0 1.96 0.107
nanoparticles Fe- -Fe 3.9 2.97 0.101
Fe- -Fe 8.5 3.43 0.105
DMSA-coated maghemite Fe-O 4.9 1.95 0.090
nanoparticles Fe-S 0.5 2.21 0.010
Fe- -Fe 4.8 2.96 0.108
Fe- -Fe 7.3 3.44 0.094