Page 399 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 399
382 Environmental Applications of Nanomaterials
3000
2500
q (µmol/g) 2000
1500
1000
As (III)
500
As (V)
0
0 100 200 300
Concentration in aqueous phase (µmol/L)
III
V
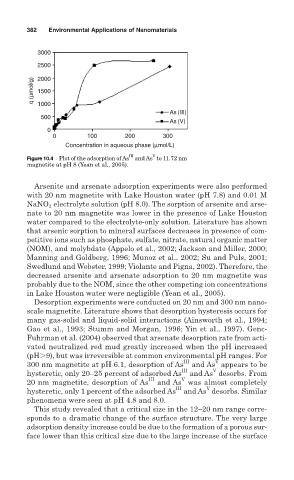
Figure 10.4 Plot of the adsorption of As and As to 11.72 nm
magnetite at pH 8 (Yean et al., 2005).
Arsenite and arsenate adsorption experiments were also performed
with 20 nm magnetite with Lake Houston water (pH 7.8) and 0.01 M
NaNO 3 electrolyte solution (pH 8.0). The sorption of arsenite and arse-
nate to 20 nm magnetite was lower in the presence of Lake Houston
water compared to the electrolyte-only solution. Literature has shown
that arsenic sorption to mineral surfaces decreases in presence of com-
petitive ions such as phosphate, sulfate, nitrate, natural organic matter
(NOM), and molybdate (Appelo et al., 2002; Jackson and Miller, 2000;
Manning and Goldberg, 1996; Munoz et al., 2002; Su and Puls, 2001;
Swedlund and Webster, 1999; Violante and Pigna, 2002). Therefore, the
decreased arsenite and arsenate adsorption to 20 nm magnetite was
probably due to the NOM, since the other competing ion concentrations
in Lake Houston water were negligible (Yean et al., 2005).
Desorption experiments were conducted on 20 nm and 300 nm nano-
scale magnetite. Literature shows that desorption hysteresis occurs for
many gas-solid and liquid-solid interactions (Ainsworth et al., 1994;
Gao et al., 1993; Stumm and Morgan, 1996; Yin et al., 1997). Genc-
Fuhrman et al. (2004) observed that arsenate desorption rate from acti-
vated neutralized red mud greatly increased when the pH increased
(pH 9), but was irreversible at common environmental pH ranges. For
V
300 nm magnetite at pH 6.1, desorption of As III and As appears to be
V
hysteretic, only 20–25 percent of adsorbed As III and As desorbs. From
V
20 nm magnetite, desorption of As III and As was almost completely
V
hysteretic, only 1 percent of the adsorbed As III and As desorbs. Similar
phenomena were seen at pH 4.8 and 8.0.
This study revealed that a critical size in the 12–20 nm range corre-
sponds to a dramatic change of the surface structure. The very large
adsorption density increase could be due to the formation of a porous sur-
face lower than this critical size due to the large increase of the surface