Page 396 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 396
Nanomaterials as Adsorbents 379
worth noting that the covalent attachment of molecules to the surface of
nanoparticles and the stability of the coating are important. In the fol-
lowing sections, we consider the role and effectiveness of nano-sized mag-
III
netic particles, including maghemite (γ-Fe O , a Fe oxide) and magnetite
2
3
II III
(Fe O , a mixed Fe and Fe oxide) in the adsorption of inorganic and
4
3
organic ions from aqueous solution, focusing on the interactions between
III V
these nanoparticles, arsenite (As ) and arsenate (As ).
Iron oxide nanoparticles for metals and
metalloids removal
Numerous papers have shown that iron oxides have a high affinity for
arsenite and arsenate (Dixit and Hering, 2003; Pierce and Moore, 1982;
Raven et al., 1998). Direct evidence of inner sphere adsorption of arsenic
to iron oxides has been provided by extended X-ray absorption fine struc-
ture and infrared spectroscopy (XAS, FTIR) spectroscopy. This adsorp-
tion is mainly controlled by the surface properties of the absorbate, the
pH on the solution, and the oxidation state of arsenic.
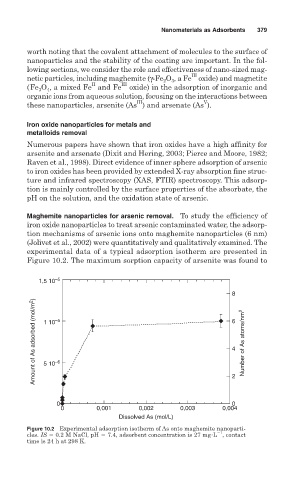
Maghemite nanoparticles for arsenic removal. To study the efficiency of
iron oxide nanoparticles to treat arsenic contaminated water, the adsorp-
tion mechanisms of arsenic ions onto maghemite nanoparticles (6 nm)
(Jolivet et al., 2002) were quantitatively and qualitatively examined. The
experimental data of a typical adsorption isotherm are presented in
Figure 10.2. The maximum sorption capacity of arsenite was found to
1,5 10 –5
8
Amount of As adsorbed (mol/m 2 ) 1 10 –5 6 4 Number of As atoms/nm 2
–6
5 10
0 2 0
0 0,001 0,002 0,003 0,004
Dissolved As (mol/L)
Figure 10.2 Experimental adsorption isotherm of As onto maghemite nanoparti-
1
cles. IS 0.2 M NaCl, pH 7.4, adsorbent concentration is 27 mg L , contact
time is 24 h at 298 K.