Page 395 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 395
378 Environmental Applications of Nanomaterials
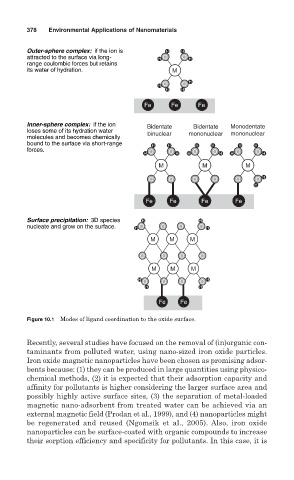
Outer-sphere complex: if the ion is H H
attracted to the surface via long- H O O H
range coulombic forces but retains
its water of hydration. M
H O O H
H H
Fe Fe Fe
Inner-sphere complex: if the ion Bidentate Bidentate Monodentate
loses some of its hydration water binuclear mononuclear mononuclear
molecules and becomes chemically
bound to the surface via short-range H H H H H H
forces.
H O O H H O O H H O O H
M M M
O O O O O O H
H
Fe Fe Fe Fe
Surface precipitation: 3D species H H
nucleate and grow on the surface. H O O O O H
M M M
O O O O
M M M
H H
O O O O
H H
Fe Fe
Figure 10.1 Modes of ligand coordination to the oxide surface.
Recently, several studies have focused on the removal of (in)organic con-
taminants from polluted water, using nano-sized iron oxide particles.
Iron oxide magnetic nanoparticles have been chosen as promising adsor-
bents because: (1) they can be produced in large quantities using physico-
chemical methods, (2) it is expected that their adsorption capacity and
affinity for pollutants is higher considering the larger surface area and
possibly highly active surface sites, (3) the separation of metal-loaded
magnetic nano-adsorbent from treated water can be achieved via an
external magnetic field (Prodan et al., 1999), and (4) nanoparticles might
be regenerated and reused (Ngomsik et al., 2005). Also, iron oxide
nanoparticles can be surface-coated with organic compounds to increase
their sorption efficiency and specificity for pollutants. In this case, it is