Page 88 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 88
74 Principles and Methods
(a) (b)
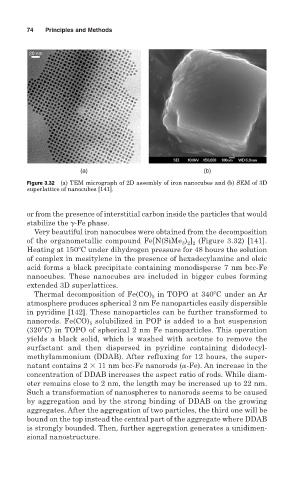
Figure 3.32 (a) TEM micrograph of 2D assembly of iron nanocubes and (b) SEM of 3D
superlattice of nanocubes [141].
or from the presence of interstitial carbon inside the particles that would
stabilize the -Fe phase.
Very beautiful iron nanocubes were obtained from the decomposition
of the organometallic compound Fe[N(SiMe ) ] (Figure 3.32) [141].
3 2 2
Heating at 150 C under dihydrogen pressure for 48 hours the solution
of complex in mesitylene in the presence of hexadecylamine and oleic
acid forms a black precipitate containing monodisperse 7 nm bcc-Fe
nanocubes. These nanocubes are included in bigger cubes forming
extended 3D superlattices.
Thermal decomposition of Fe(CO) in TOPO at 340 C under an Ar
5
atmosphere produces spherical 2 nm Fe nanoparticles easily dispersible
in pyridine [142]. These nanoparticles can be further transformed to
nanorods. Fe(CO) solubilized in POP is added to a hot suspension
5
(320 C) in TOPO of spherical 2 nm Fe nanoparticles. This operation
yields a black solid, which is washed with acetone to remove the
surfactant and then dispersed in pyridine containing didodecyl-
methylammonium (DDAB). After refluxing for 12 hours, the super-
natant contains 2 11 nm bcc-Fe nanorods ( -Fe). An increase in the
concentration of DDAB increases the aspect ratio of rods. While diam-
eter remains close to 2 nm, the length may be increased up to 22 nm.
Such a transformation of nanospheres to nanorods seems to be caused
by aggregation and by the strong binding of DDAB on the growing
aggregates. After the aggregation of two particles, the third one will be
bound on the top instead the central part of the aggregate where DDAB
is strongly bounded. Then, further aggregation generates a unidimen-
sional nanostructure.