Page 92 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 92
78 Principles and Methods
the sheet from being planar. The unusual structure of C led to the intro-
60
duction of a new class of molecules known as fullerenes, which now con-
stitute the third allotrope of carbon. Fullerenes are commonly defined
as “any of a class of closed hollow aromatic carbon compounds that are
made up of twelve pentagonal and differing numbers of hexagonal faces.”
The number of carbon atoms in a fullerene range from C 60 to C , C ,
70
76
and higher. Higher order fullerenes include carbon nanotubes that can
be described as fullerenes that have been stretched along a rotational
axis to form a tube. Given the differences in the chemistry and size of
fullerenes such as C and C 70 as compared to nanotubes, these will be
60
dealt with separately. However, it should be appreciated that they are
all part of the fullerene allotrope of carbon. In addition there have also
been reports of nanohorns [149] and nanofibers [150]. Nanohorns are
single-walled carbon cones that can be filled with biological material or
metal oxides.
Fullerenes and carbon nanotubes are not necessarily products of high-
tech laboratories; they are commonly formed in such mundane places
as ordinary flames, produced by burning hydrocarbons [151, 152], and
they have been found in soot from both indoor and outdoor air [153].
However, these naturally occurring varieties can be highly irregular in
size and quality because the environment in which they are produced
is often highly uncontrolled. Thus, although they can be used in some
applications, they can lack in the high degree of uniformity necessary
to meet many needs of both research and industry.
Fullerenes
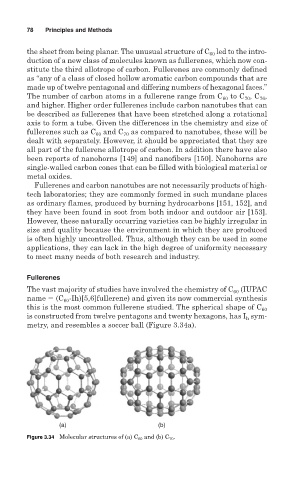
The vast majority of studies have involved the chemistry of C (IUPAC
60
name (C -Ih)[5,6]fullerene) and given its now commercial synthesis
60
this is the most common fullerene studied. The spherical shape of C 60
is constructed from twelve pentagons and twenty hexagons, has I sym-
h
metry, and resembles a soccer ball (Figure 3.34a).
(a) (b)
Figure 3.34 Molecular structures of (a) C 60 and (b) C 70 .