Page 94 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 94
80 Principles and Methods
symmetry of C , these molecules provide prototype systems for spec-
60
troscopy, optics, and other basic science investigations.
Synthesis of fullerenes. The first observation of fullerenes was in molec-
ular beam experiments, where discrete peaks were observed correspon-
ding to molecules with the exact mass of 60 or 70 or more carbon atoms.
In 1985, Harold Kroto (of the University of Sussex), James R. Heath,
Sean O’Brien, Robert Curl, and Richard Smalley (from Rice University)
published their observations along with the proposed structure for C 60
[1]. Subsequent studies demonstrated that C was in fact ubiquitous in
60
carbon combustion, and by 1991 it was relatively easy to produce grams
of fullerene powder. Although the synthesis is relatively straightfor-
ward, fullerene purification remains a challenge to chemists and deter-
mines fullerene prices to a large extent.
The first method of production of fullerenes used laser vaporization of
carbon in an inert atmosphere, but this produced microscopic amounts of
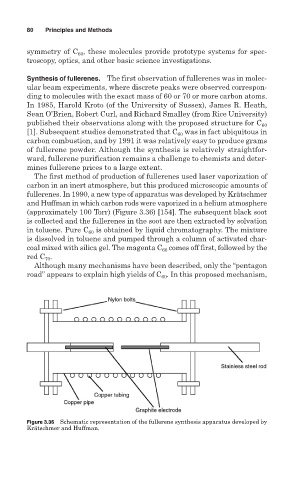
fullerenes. In 1990, a new type of apparatus was developed by Krätschmer
and Huffman in which carbon rods were vaporized in a helium atmosphere
(approximately 100 Torr) (Figure 3.36) [154]. The subsequent black soot
is collected and the fullerenes in the soot are then extracted by solvation
in toluene. Pure C is obtained by liquid chromatography. The mixture
60
is dissolved in toluene and pumped through a column of activated char-
coal mixed with silica gel. The magenta C comes off first, followed by the
60
red C .
70
Although many mechanisms have been described, only the “pentagon
road” appears to explain high yields of C . In this proposed mechanism,
60
Figure 3.36 Schematic representation of the fullerene synthesis apparatus developed by
Krätschmer and Huffman.