Page 99 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 99
Nanomaterials Fabrication 85
EtO(O)C C(O)OEt
C(O)OEt
Br + NaH
C(O)OEt
–H 2 , –NaBr
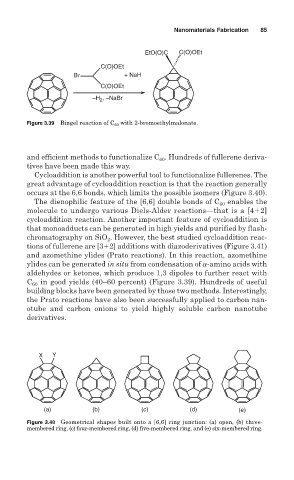
Figure 3.39 Bingel reaction of C 60 with 2-bromoethylmalonate.
and efficient methods to functionalize C . Hundreds of fullerene deriva-
60
tives have been made this way.
Cycloaddition is another powerful tool to functionalize fullerenes. The
great advantage of cycloaddition reaction is that the reaction generally
occurs at the 6,6 bonds, which limits the possible isomers (Figure 3.40).
The dienophilic feature of the [6,6] double bonds of C 60 enables the
molecule to undergo various Diels-Alder reactions—that is a [4 2]
cycloaddition reaction. Another important feature of cycloaddition is
that monoadducts can be generated in high yields and purified by flash-
chromatography on SiO . However, the best studied cycloaddition reac-
2
tions of fullerene are [3 2] additions with diazoderivatives (Figure 3.41)
and azomethine ylides (Prato reactions). In this reaction, azomethine
ylides can be generated in situ from condensation of -amino acids with
aldehydes or ketones, which produce 1,3 dipoles to further react with
C 60 in good yields (40–60 percent) (Figure 3.39). Hundreds of useful
building blocks have been generated by those two methods. Interestingly,
the Prato reactions have also been successfully applied to carbon nan-
otube and carbon onions to yield highly soluble carbon nanotube
derivatives.
X Y
(a) (b) (c) (d) (e)
Figure 3.40 Geometrical shapes built onto a [6,6] ring junction: (a) open, (b) three-
membered ring, (c) four-membered ring, (d) five-membered ring, and (e) six-membered ring.