Page 100 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 100
86 Principles and Methods
CH 3
N
O
CH 3
H ∆ + C 60
+ H C = O
N 2 N +
H C OH –CO 2 CH –
3
2
–H O H C 2
2
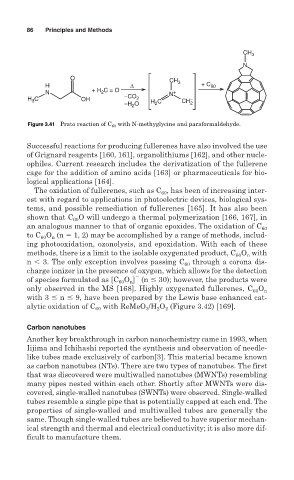
Figure 3.41 Prato reaction of C 60 with N-methyglycine and paraformaldehyde.
Successful reactions for producing fullerenes have also involved the use
of Grignard reagents [160, 161], organolithiums [162], and other nucle-
ophiles. Current research includes the derivatization of the fullerene
cage for the addition of amino acids [163] or pharmaceuticals for bio-
logical applications [164].
The oxidation of fullerenes, such as C , has been of increasing inter-
60
est with regard to applications in photoelectric devices, biological sys-
tems, and possible remediation of fullerenes [165]. It has also been
shown that C O will undergo a thermal polymerization [166, 167], in
60
an analogous manner to that of organic epoxides. The oxidation of C 60
to C O (n 1, 2) may be accomplished by a range of methods, includ-
n
60
ing photooxidation, ozonolysis, and epoxidation. With each of these
methods, there is a limit to the isolable oxygenated product, C O with
60
n
n 3. The only exception involves passing C 60 through a corona dis-
charge ionizer in the presence of oxygen, which allows for the detection
of species formulated as [C O ] (n 30); however, the products were
60
n
only observed in the MS [168]. Highly oxygenated fullerenes, C O n
60
with 3 n 9, have been prepared by the Lewis base enhanced cat-
alytic oxidation of C 60 with ReMeO /H O (Figure 3.42) [169].
2
3
2
Carbon nanotubes
Another key breakthrough in carbon nanochemistry came in 1993, when
Iijima and Ichihashi reported the synthesis and observation of needle-
like tubes made exclusively of carbon[3]. This material became known
as carbon nanotubes (NTs). There are two types of nanotubes. The first
that was discovered were multiwalled nanotubes (MWNTs) resembling
many pipes nested within each other. Shortly after MWNTs were dis-
covered, single-walled nanotubes (SWNTs) were observed. Single-walled
tubes resemble a single pipe that is potentially capped at each end. The
properties of single-walled and multiwalled tubes are generally the
same. Though single-walled tubes are believed to have superior mechan-
ical strength and thermal and electrical conductivity; it is also more dif-
ficult to manufacture them.