Page 96 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 96
82 Principles and Methods
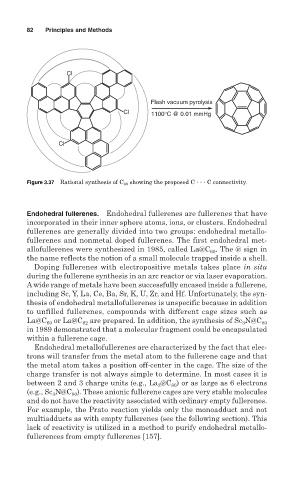
Cl
Flash vacuum pyrolysis
Cl 1100°C @ 0.01 mmHg
Cl
Figure 3.37 Rational synthesis of C 60 showing the proposed C C connectivity.
Endohedral fullerenes. Endohedral fullerenes are fullerenes that have
incorporated in their inner sphere atoms, ions, or clusters. Endohedral
fullerenes are generally divided into two groups: endohedral metallo-
fullerenes and nonmetal doped fullerenes. The first endohedral met-
allofullerenes were synthesized in 1985, called La@C . The @ sign in
60
the name reflects the notion of a small molecule trapped inside a shell.
Doping fullerenes with electropositive metals takes place in situ
during the fullerene synthesis in an arc reactor or via laser evaporation.
A wide range of metals have been successfully encased inside a fullerene,
including Sc, Y, La, Ce, Ba, Sr, K, U, Zr, and Hf. Unfortunately, the syn-
thesis of endohedral metallofullerenes is unspecific because in addition
to unfilled fullerenes, compounds with different cage sizes such as
La@C or La@C are prepared. In addition, the synthesis of Sc N@C 80
60
82
3
in 1989 demonstrated that a molecular fragment could be encapsulated
within a fullerene cage.
Endohedral metallofullerenes are characterized by the fact that elec-
trons will transfer from the metal atom to the fullerene cage and that
the metal atom takes a position off-center in the cage. The size of the
charge transfer is not always simple to determine. In most cases it is
between 2 and 3 charge units (e.g., La @C ) or as large as 6 electrons
80
2
(e.g., Sc N@C ). These anionic fullerene cages are very stable molecules
3
80
and do not have the reactivity associated with ordinary empty fullerenes.
For example, the Prato reaction yields only the monoadduct and not
multiadducts as with empty fullerenes (see the following section). This
lack of reactivity is utilized in a method to purify endohedral metallo-
fullerences from empty fullerenes [157].