Page 20 - Essentials of physical chemistry
P. 20
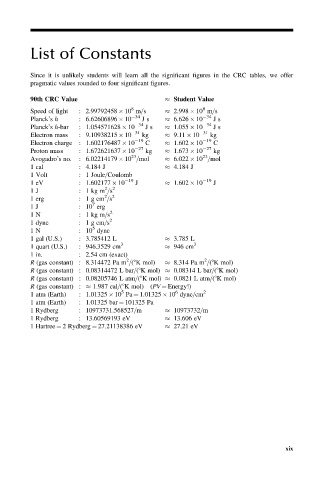
List of Constants
Since it is unlikely students will learn all the significant figures in the CRC tables, we offer
pragmatic values rounded to four significant figures.
90th CRC Value Student Value
8
8
Speed of light : 2.99792458 10 m=s 2.998 10 m=s
Planck’s h : 6.62606896 10 34 Js 6.626 10 34 Js
Planck’s h-bar : 1.054571628 10 34 Js 1.055 10 34 Js
Electron mass : 9.10938215 10 31 kg 9.11 10 31 kg
Electron charge : 1.602176487 10 19 C 1.602 10 19 C
Proton mass : 1.672621637 10 27 kg 1.673 10 27 kg
23
23
Avogadro’s no. : 6.02214179 10 =mol 6.022 10 =mol
1 cal : 4.184 J 4.184 J
1 Volt : 1 Joule=Coulomb
1 eV : 1.602177 10 19 J 1.602 10 19 J
2
1 J : 1 kg m =s 2
2
1 erg : 1 g cm =s 2
7
1J : 10 erg
1 N : 1 kg m=s 2
2
1 dyne : 1 g cm=s
5
1N : 10 dyne
1 gal (U.S.) : 3.785412 L 3.785 L
3 3
1 quart (U.S.) : 946.3529 cm 946 cm
1 in. : 2.54 cm (exact)
2
2
R (gas constant) : 8.314472 Pa m =(8K mol) 8.314 Pa m =(8K mol)
R (gas constant) : 0.08314472 L bar=(8K mol) 0.08314 L bar=(8K mol)
R (gas constant) : 0.08205746 L atm=(8K mol) 0.0821 L atm=(8K mol)
R (gas constant) : 1.987 cal=(8K mol) (PV ¼ Energy!)
6
5
1 atm (Earth) : 1.01325 10 Pa ¼ 1.01325 10 dyne=cm 2
1 atm (Earth) : 1.01325 bar ¼ 101325 Pa
1 Rydberg : 10973731.568527=m 10973732=m
1 Rydberg : 13.60569193 eV 13.606 eV
1 Hartree ¼ 2 Rydberg ¼ 27.21138386 eV 27.21 eV
xix