Page 259 - Essentials of physical chemistry
P. 259
Early Experiments in Quantum Physics 221
hn
Thus we have lim 1 ¼ 0. So the amazing thing about Planck’s average energy
hn ¼
e B T 1
n!1 k e 1
expression is that it goes to zero at the high frequency end and is asymptotic to the experimental curve
at the low frequency end! We have indulged in your patience with the tedium of the derivation because
it is exact! Energy is quantized!
One additional amazing confirmation is that we can integrate Planck’s formula over all frequen-
cies to obtain the total emissive power to compare to the Stefan–Boltzmann law
4
ð ð 3 ð 3
8ph n dn 8ph k B T x dx hn
1 1 1
. How-
r(n)dn ¼ 3 hn ¼ 3 x by substituting x ¼
0 c 0 e B T 1 c h 0 (e 1) k B T
k
ever, this involves a very difficult integral, which can be solved by an expansion technique
ð 3 4
x dx p
1
and term-by-term integration but it is readily found in a table of integrals as x ¼ .
0 (e 1) 15
ð 4 4 5 4
8ph k B T p 8p k B 4 4
1
Thus we can derive r(n)dn ¼ 3 ¼ 3 3 T ¼ aT , which agrees
0 c h 15 15h c
with experiment!
PHOTOELECTRIC EFFECT
Once again we invoke our strategy of not discussing all of the amazing discoveries in the early
1900s but focusing on the ‘‘essential’’ facts. Some biographies of Albert Einstein reveal that perhaps
he was the foremost advocate of the idea of tiny atoms in nature, agreeing with Boltzmann.
Throughout the 1800s, there were a number of experiments that showed that light could knock
electrons out of a metal surface. The strange thing about the effect was that it seemed to depend on
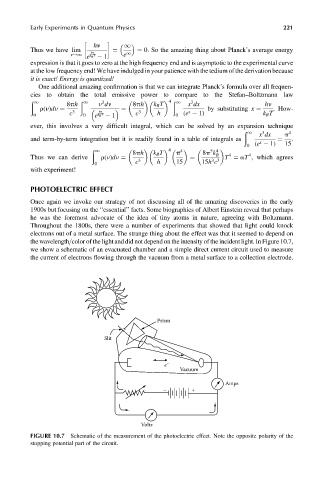
the wavelength=color of the light and did not depend on the intensity of the incident light. In Figure 10.7,
we show a schematic of an evacuated chamber and a simple direct current circuit used to measure
the current of electrons flowing through the vacuum from a metal surface to a collection electrode.
Prism
Slit
e –
Vacuum
Amps
– +
Volts
FIGURE 10.7 Schematic of the measurement of the photoelectric effect. Note the opposite polarity of the
stopping potential part of the circuit.