Page 50 - Essentials of physical chemistry
P. 50
12 Essentials of Physical Chemistry
By Dalton’s law of partial pressures the total pressure of 750 mmHg of the gas trapped over the
water is the sum of the pressure of the water vapor and that of the N 2 so we have
¼ 750 mmHg P H 2 O ¼ (750 21:06)mmHg ¼ 728:92 mmHg:
P N 2
Then we can calculate the moles of N 2 from the ideal gas equation.
2 3
728:92 mmHg
(0:450 L)
PV 6 760 mmHg=atm 7
6 7 ffi 0:0178 mol N 2 :
n ¼ ¼
RT 4 0:08206 L atm= K mol (273:15 þ 23) K
5
This hypothetical problem is given here to illustrate the use of Dalton’s law of partial pressures and to
discuss the process of uncertainty analysis, which may be important if a forensic laboratory technician
has to testify in a court case or a chemistry student has to write a report for a laboratory course.
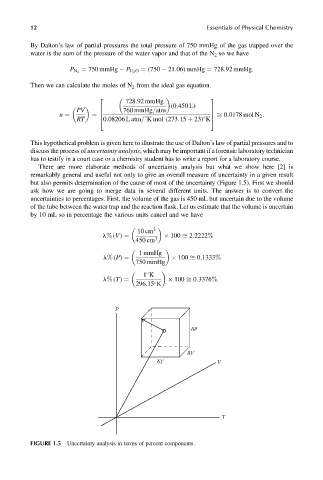
There are more elaborate methods of uncertainty analysis but what we show here [2] is
remarkably general and useful not only to give an overall measure of uncertainty in a given result
but also permits determination of the cause of most of the uncertainty (Figure 1.5). First we should
ask how we are going to merge data in several different units. The answer is to convert the
uncertainties to percentages. First, the volume of the gas is 450 mL but uncertain due to the volume
of the tube between the water trap and the reaction flask. Let us estimate that the volume is uncertain
by 10 mL so in percentage the various units cancel and we have
3
10 cm
100 ffi 2:2222%
450 cm
l% (V) ¼ 3
1 mmHg
100 ffi 0:1333%
750 mmHg
l% (P) ¼
1 K
100 ffi 0:3376%:
l% (T) ¼
296:15 K
P
δP
δV
δT V
T
FIGURE 1.5 Uncertainty analysis in terms of percent components.