Page 172 - Facility Piping Systems Handbook for Industrial, Commercial, and Healthcare Facilities
P. 172
WATER TREATMENT AND PURIFICATION
4.8 CHAPTER FOUR
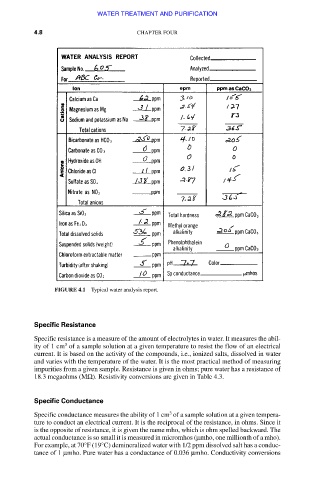
FIGURE 4.1 Typical water analysis report.
Specific Resistance
Specific resistance is a measure of the amount of electrolytes in water. It measures the abil-
3
ity of 1 cm of a sample solution at a given temperature to resist the flow of an electrical
current. It is based on the activity of the compounds, i.e., ionized salts, dissolved in water
and varies with the temperature of the water. It is the most practical method of measuring
impurities from a given sample. Resistance is given in ohms; pure water has a resistance of
18.3 megaohms (MΩ). Resistivity conversions are given in Table 4.3.
Specific Conductance
3
Specific conductance measures the ability of 1 cm of a sample solution at a given tempera-
ture to conduct an electrical current. It is the reciprocal of the resistance, in ohms. Since it
is the opposite of resistance, it is given the name mho, which is ohm spelled backward. The
actual conductance is so small it is measured in micromhos (μmho, one millionth of a mho).
For example, at 70°F (19°C) demineralized water with 1/2 ppm dissolved salt has a conduc-
tance of 1 μmho. Pure water has a conductance of 0.036 μmho. Conductivity conversions
Downloaded from Digital Engineering Library @ McGraw-Hill (www.accessengineeringlibrary.com)
Copyright © 2009 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.