Page 179 - Fiber Fracture
P. 179
164 J.G. Lavin
Initially, commercial PAN-based carbon fibers were made from the polymers devel-
oped for textile applications. However, these fibers were neither very stiff nor strong.
Development efforts over the 1960s and 1970s focused on increasing molecular weight,
introducing co-monomers to assist processing, and eliminating impurities which limited
mechanical strength. The chemistry of conversion of PAN to carbon is quite complex,
and the interested reader is referred to an excellent treatment in Peebles (1994). The
critical steps are outlined below.
The first critical step in making carbon fiber from PAN fiber is causing the pendant
nitrile groups to cyclize, as illustrated in Fig. 8. This process is thermally activated and
is highly exothermic. The activation temperature is influenced by the type and amount
of co-monomer used. It is also important to keep the fiber under tension in this process,
and indeed, during the whole conversion process. The next step is to make the fiber
infusible: this is accomplished by adding oxygen atoms to the polymer, again by heating
in air. The reaction is diffusion limited, requiring exposure times of tens of minutes.
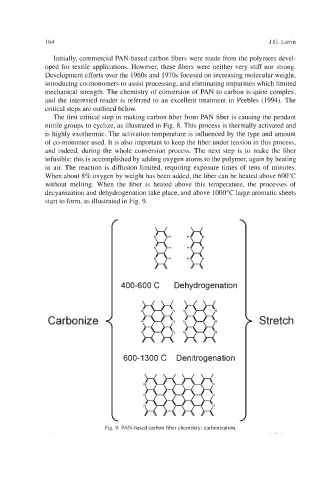
When about 8% oxygen by weight has been added, the fiber can be heated above 600°C
without melting. When the fiber is heated above this temperature, the processes of
decyanization and dehydrogenation take place, and above 1000°C large aromatic sheets
start to form, as illustrated in Fig. 9.
H H
H H
H H
00-600 C Dehydrogenation
Carbonize + Stretch
600-1 300 C Denitrogenation
Fig. 9. PAN-based carbon fiber chemistry: carbonization.