Page 332 - Fiber Fracture
P. 332
3 14 C. Viney
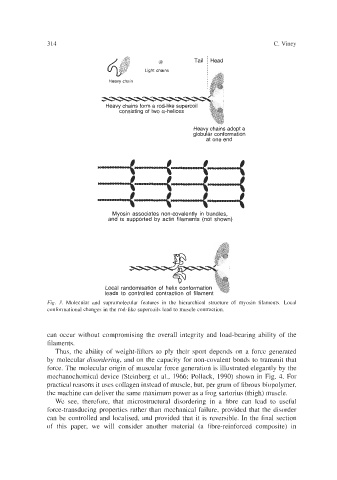
Heavy chains form a rod-like supercoil
consisting of two a-helices
..,.,_,.
....I.I .\
. .. .
%;::?j:
Heavy chains adopt a
globular conformation
at one end
Myosin associates non-covalently in bundles,
and is supported by actin filaments (not shown)
. ..
Local randomisation of helix conformation
leads to controlled contraction of filament
Fig. 3. Molecular and supramolecular features in the hierarchical structure of myosin filaments. Local
conformational changes in the rod-like supercoils lead to muscle contraction.
can occur without compromising the overall integrity and load-bearing ability of the
filaments.
Thus, the ability of weight-lifters to ply their sport depends on a force generated
by molecular disordering, and on the capacity for non-covalent bonds to transmit that
force. The molecular origin of muscular force generation is illustrated elegantly by the
mechanochemical device (Steinberg et al., 1966; Pollack, 1990) shown in Fig. 4. For
practical reasons it uses collagen instead of muscle, but, per gram of fibrous biopolymer,
the machine can deliver the same maximum power as a frog sartorius (thigh) muscle.
We see, therefore, that microstructural disordering in a fibre can lead to useful
force-transducing properties rather than mechanical failure, provided that the disorder
can be controlled and localised, and provided that it is reversible. In the final section
of this paper, we will consider another material (a fibre-reinforced composite) in