Page 64 - Fluid Catalytic Cracking Handbook
P. 64
42 Fluid Catalytic Cracking Handbook
Olefins
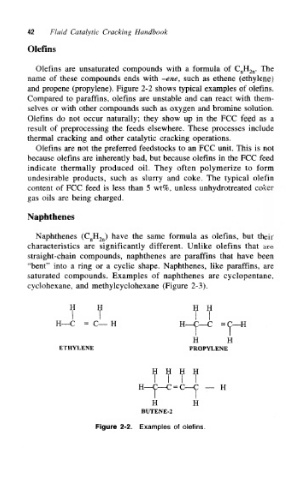
Olefins are unsaturated compounds with a formula of C nH 2n. The
name of these compounds ends with ~ene, such as ethene (ethylene)
and propene (propylene). Figure 2-2 shows typical examples of olefins.
Compared to paraffins, olefins are unstable and can react with them-
selves or with other compounds such as oxygen and bromine solution.
Olefins do not occur naturally; they show up in the FCC feed as a
result of preprocessing the feeds elsewhere. These processes include
thermal cracking and other catalytic cracking operations.
Olefins are not the preferred feedstocks to an FCC unit. This is not
because olefins are inherently bad, but because olefins in the FCC feed
indicate thermally produced oil. They often polymerize to form
undesirable products, such as slurry and coke. The typical olefin
content of FCC feed is less than 5 wt%, unless unhydrotreated coker
gas oils are being charged.
Napfathenes
Naptithenes (C irH 2n) have the same formula as olefins, but their
characteristics are significantly different. Unlike olefins that are
straight-chain compounds, naphthenes are paraffins that have been
"bent" into a ring or a cyclic shape. Naphthenes, like paraffins, are
saturated compounds. Examples of naphthenes are cyclopentane,
cyclohexane, and methylcyclohexane (Figure 2-3).
H H H H
H—C = C—H H—C—C =C—H
H H
ETHYLENE PROPYLENE
m?
c = c—c — H
H H
BUTENE-2
Figure 2-2, Examples of olefins.