Page 81 - Fluid Catalytic Cracking Handbook
P. 81
FCC Feed Characterization, 59
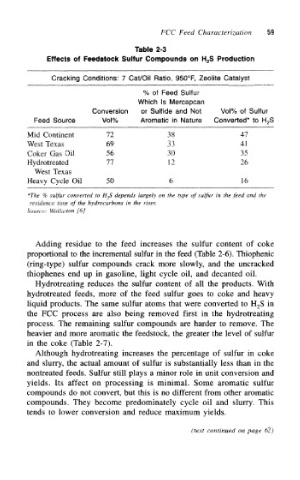
Table 2-3
Effects of Feedstock Sulfur Compounds on H 2S Production
Cracking Conditions: 7 Cat/Oil Ratio, 950°F, Zeolite Catalyst
% of Feed Sulfur
Which Is Mercapcan
Conversion or Sulfide and Not Vol% of Sulfur
Feed Source Vol% Aromatic in Nature Converted* to H 2S
Mid Continent 72 38 47
West Texas 69 33 4!
Coker Gas Oil 56 30 35
77
Hydrotreated 12 26
West Texas
Heavy Cycle Oil 50 6 16
*The % sulfur converted to H-,S depends largely on the type of sulfur in the feed and the
residence time of the hydrocarbons in the riser.
Source: Wollaston [6]
Adding residue to the feed increases the sulfur content of coke
proportional to the incremental sulfur in the feed (Table 2-6). Thiophenic
(ring-type) sulfur compounds crack more slowly, and the uncracked
thiophenes end up in gasoline, light cycle oil, and decanted oil.
Hydrotreating reduces the sulfur content of all the products. With
hydrotreated feeds, more of the feed sulfur goes to coke and heavy
liquid products. The same sulfur atoms that were converted to H 2S in
the FCC process are also being removed first in the hydrotreating
process. The remaining sulfur compounds are harder to remove. The
heavier and more aromatic the feedstock, the greater the level of sulfur
in the coke (Table 2-7).
Although hydrotreating increases the percentage of sulfur in coke
and slurry, the actual amount of sulfur is substantially less than in the
nontreated feeds. Sulfur still plays a minor role in unit conversion and
yields. Its affect on processing is minimal. Some aromatic sulfur
compounds do not convert, but this is no different from other aromatic
compounds. They become predominately cycle oil and slurry. This
tends to lower conversion and reduce maximum yields.
(text continued on page 62)