Page 32 - Instant notes
P. 32
Physical Chemistry 18
positions. They are better described in terms of a radial distribution function, since there
is only the probability of finding a neighbor at a given radial distance, rather than the
certainty of a neighbor at a fixed point.
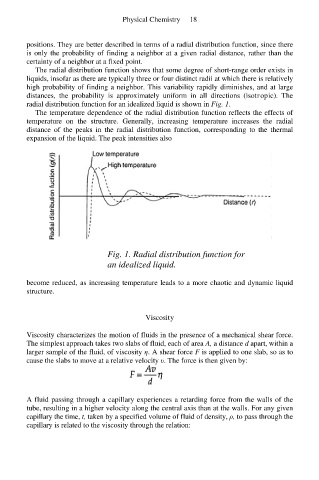
The radial distribution function shows that some degree of short-range order exists in
liquids, insofar as there are typically three or four distinct radii at which there is relatively
high probability of finding a neighbor. This variability rapidly diminishes, and at large
distances, the probability is approximately uniform in all directions (isotropic). The
radial distribution function for an idealized liquid is shown in Fig. 1.
The temperature dependence of the radial distribution function reflects the effects of
temperature on the structure. Generally, increasing temperature increases the radial
distance of the peaks in the radial distribution function, corresponding to the thermal
expansion of the liquid. The peak intensities also
Fig. 1. Radial distribution function for
an idealized liquid.
become reduced, as increasing temperature leads to a more chaotic and dynamic liquid
structure.
Viscosity
Viscosity characterizes the motion of fluids in the presence of a mechanical shear force.
The simplest approach takes two slabs of fluid, each of area A, a distance d apart, within a
larger sample of the fluid, of viscosity η. A shear force F is applied to one slab, so as to
cause the slabs to move at a relative velocity υ. The force is then given by:
A fluid passing through a capillary experiences a retarding force from the walls of the
tube, resulting in a higher velocity along the central axis than at the walls. For any given
capillary the time, t, taken by a specified volume of fluid of density, ρ, to pass through the
capillary is related to the viscosity through the relation: