Page 35 - Instant notes
P. 35
Liquids 21
Liquid crystals
A material may melt from the crystalline state (see Topic A5) into a superficially liquid
state, and yet retain most of the short-range order, and some of the long-range order so
that it cannot be considered to be a true liquid. Such materials are neither wholly solid
nor wholly liquid, and are termed liquid crystals. Liquid crystals tend to be formed from
molecules which are highly anisotropic, with rod, disk, or other similar shapes. Several
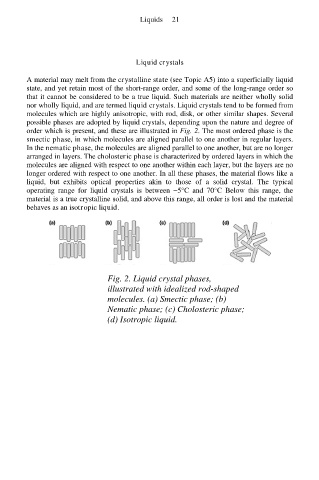
possible phases are adopted by liquid crystals, depending upon the nature and degree of
order which is present, and these are illustrated in Fig. 2. The most ordered phase is the
smectic phase, in which molecules are aligned parallel to one another in regular layers.
In the nematic phase, the molecules are aligned parallel to one another, but are no longer
arranged in layers. The cholosteric phase is characterized by ordered layers in which the
molecules are aligned with respect to one another within each layer, but the layers are no
longer ordered with respect to one another. In all these phases, the material flows like a
liquid, but exhibits optical properties akin to those of a solid crystal. The typical
operating range for liquid crystals is between −5°C and 70°C Below this range, the
material is a true crystalline solid, and above this range, all order is lost and the material
behaves as an isotropic liquid.
Fig. 2. Liquid crystal phases,
illustrated with idealized rod-shaped
molecules. (a) Smectic phase; (b)
Nematic phase; (c) Cholosteric phase;
(d) Isotropic liquid.