Page 41 - Instant notes
P. 41
Crystalline solids 27
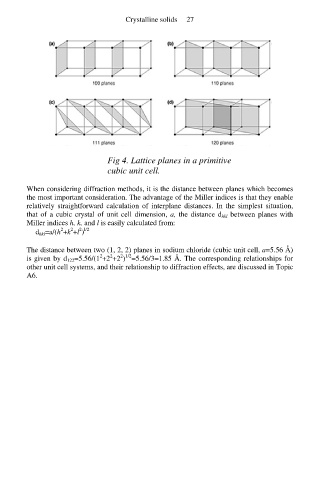
Fig 4. Lattice planes in a primitive
cubic unit cell.
When considering diffraction methods, it is the distance between planes which becomes
the most important consideration. The advantage of the Miller indices is that they enable
relatively straightforward calculation of interplane distances. In the simplest situation,
that of a cubic crystal of unit cell dimension, a, the distance d hkl between planes with
Miller indices h, k, and l is easily calculated from:
2
2
2 l/2
d hkl=a/(h +k +l )
The distance between two (1, 2, 2) planes in sodium chloride (cubic unit cell, a=5.56 Å)
2
2 1/2
2
is given by d 122=5.56/(1 +2 +2 ) =5.56/3=1.85 Å. The corresponding relationships for
other unit cell systems, and their relationship to diffraction effects, are discussed in Topic
A6.