Page 44 - Instant notes
P. 44
Physical Chemistry 30
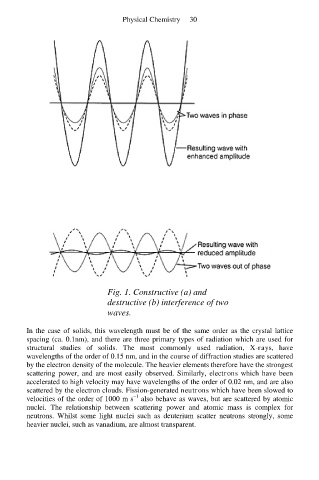
Fig. 1. Constructive (a) and
destructive (b) interference of two
waves.
In the case of solids, this wavelength must be of the same order as the crystal lattice
spacing (ca. 0.1nm), and there are three primary types of radiation which are used for
structural studies of solids. The most commonly used radiation, X-rays, have
wavelengths of the order of 0.15 nm, and in the course of diffraction studies are scattered
by the electron density of the molecule. The heavier elements therefore have the strongest
scattering power, and are most easily observed. Similarly, electrons which have been
accelerated to high velocity may have wavelengths of the order of 0.02 nm, and are also
scattered by the electron clouds. Fission-generated neutrons which have been slowed to
−1
velocities of the order of 1000 m s also behave as waves, but are scattered by atomic
nuclei. The relationship between scattering power and atomic mass is complex for
neutrons. Whilst some light nuclei such as deuterium scatter neutrons strongly, some
heavier nuclei, such as vanadium, are almost transparent.