Page 246 - Formation Damage during Improved Oil Recovery Fundamentals and Applications
P. 246
218 Xingru Wu
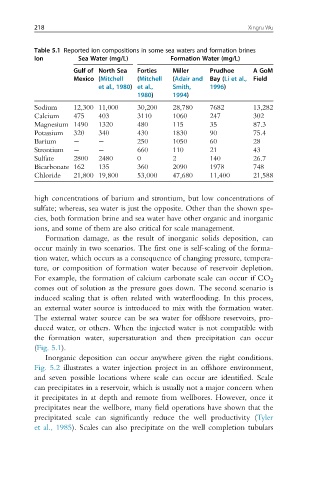
Table 5.1 Reported ion compositions in some sea waters and formation brines
Ion Sea Water (mg/L) Formation Water (mg/L)
Gulf of North Sea Forties Miller Prudhoe A GoM
Mexico (Mitchell (Mitchell (Adair and Bay (Li et al., Field
et al., 1980) et al., Smith, 1996)
1980) 1994)
Sodium 12,300 11,000 30,200 28,780 7682 13,282
Calcium 475 403 3110 1060 247 302
Magnesium 1490 1320 480 115 35 87.3
Potassium 320 340 430 1830 90 75.4
Barium 250 1050 60 28
Strontium 660 110 21 43
Sulfate 2800 2480 0 2 140 26.7
Bicarbonate 162 135 360 2090 1978 748
Chloride 21,800 19,800 53,000 47,680 11,400 21,588
high concentrations of barium and strontium, but low concentrations of
sulfate; whereas, sea water is just the opposite. Other than the shown spe-
cies, both formation brine and sea water have other organic and inorganic
ions, and some of them are also critical for scale management.
Formation damage, as the result of inorganic solids deposition, can
occur mainly in two scenarios. The first one is self-scaling of the forma-
tion water, which occurs as a consequence of changing pressure, tempera-
ture, or composition of formation water because of reservoir depletion.
For example, the formation of calcium carbonate scale can occur if CO 2
comes out of solution as the pressure goes down. The second scenario is
induced scaling that is often related with waterflooding. In this process,
an external water source is introduced to mix with the formation water.
The external water source can be sea water for offshore reservoirs, pro-
duced water, or others. When the injected water is not compatible with
the formation water, supersaturation and then precipitation can occur
(Fig. 5.1).
Inorganic deposition can occur anywhere given the right conditions.
Fig. 5.2 illustrates a water injection project in an offshore environment,
and seven possible locations where scale can occur are identified. Scale
can precipitates in a reservoir, which is usually not a major concern when
it precipitates in at depth and remote from wellbores. However, once it
precipitates near the wellbore, many field operations have shown that the
precipitated scale can significantly reduce the well productivity (Tyler
et al., 1985). Scales can also precipitate on the well completion tubulars