Page 265 - Formation Damage during Improved Oil Recovery Fundamentals and Applications
P. 265
236 Xingru Wu
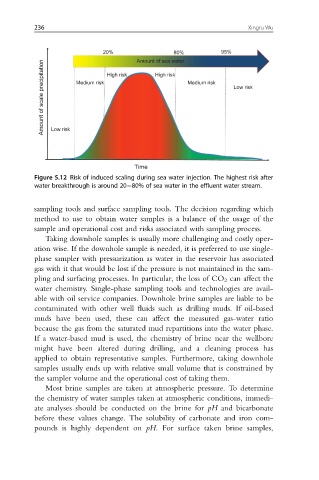
80%
20% Amount of sea water 95%
Amount of scale precipitation Low risk Medium risk Medium risk Low risk
High risk
High risk
Time
Figure 5.12 Risk of induced scaling during sea water injection. The highest risk after
water breakthrough is around 20 80% of sea water in the effluent water stream.
sampling tools and surface sampling tools. The decision regarding which
method to use to obtain water samples is a balance of the usage of the
sample and operational cost and risks associated with sampling process.
Taking downhole samples is usually more challenging and costly oper-
ation wise. If the downhole sample is needed, it is preferred to use single-
phase sampler with pressurization as water in the reservoir has associated
gas with it that would be lost if the pressure is not maintained in the sam-
pling and surfacing processes. In particular, the loss of CO 2 can affect the
water chemistry. Single-phase sampling tools and technologies are avail-
able with oil service companies. Downhole brine samples are liable to be
contaminated with other well fluids such as drilling muds. If oil-based
muds have been used, these can affect the measured gas-water ratio
because the gas from the saturated mud repartitions into the water phase.
If a water-based mud is used, the chemistry of brine near the wellbore
might have been altered during drilling, and a cleaning process has
applied to obtain representative samples. Furthermore, taking downhole
samples usually ends up with relative small volume that is constrained by
the sampler volume and the operational cost of taking them.
Most brine samples are taken at atmospheric pressure. To determine
the chemistry of water samples taken at atmospheric conditions, immedi-
ate analyses should be conducted on the brine for pH and bicarbonate
before these values change. The solubility of carbonate and iron com-
pounds is highly dependent on pH. For surface taken brine samples,