Page 204 - Fundamentals of Air Pollution 3E
P. 204
170 12. Atmospheric Chemistry
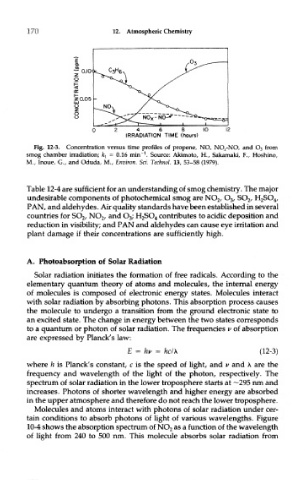
Fig. 12-3. Concentration versus time profiles of propene, NO, NO^-NO, and O 3 from
1
smog chamber irradiation; fcj = 0.16 min" . Source: Akimoto, H., Sakamaki, ¥., Hoshino,
M, Inoue, G., and Oduda, M, Environ. Sci. Technol. 13, 53-58 (1979).
Table 12-4 are sufficient for an understanding of smog chemistry. The major
undesirable components of photochemical smog are NO 2, O 3/ SO 2 , H 2SO 4,
PAN, and aldehydes. Air quality standards have been established in several
countries for SO 2 , NO 2, and O 3; H 2SO 4 contributes to acidic deposition and
reduction in visibility; and PAN and aldehydes can cause eye irritation and
plant damage if their concentrations are sufficiently high.
A. Photoabsorption of Solar Radiation
Solar radiation initiates the formation of free radicals. According to the
elementary quantum theory of atoms and molecules, the internal energy
of molecules is composed of electronic energy states. Molecules interact
with solar radiation by absorbing photons. This absorption process causes
the molecule to undergo a transition from the ground electronic state to
an excited state. The change in energy between the two states corresponds
to a quantum or photon of solar radiation. The frequencies v of absorption
are expressed by Planck's law:
where h is Planck's constant, c is the speed of light, and v and X are the
frequency and wavelength of the light of the photon, respectively. The
spectrum of solar radiation in the lower troposphere starts at ~295 nrn and
increases. Photons of shorter wavelength and higher energy are absorbed
in the upper atmosphere and therefore do not reach the lower troposphere.
Molecules and atoms interact with photons of solar radiation under cer-
tain conditions to absorb photons of light of various wavelengths. Figure
10-4 shows the absorption spectrum of NO 2 as a function of the wavelength
of light from 240 to 500 nm. This molecule absorbs solar radiation from