Page 208 - Fundamentals of Air Pollution 3E
P. 208
174 12. Atmospheric Chemistry
permit the NO 2/NO ratio to build up and steady-state O 3 concentrations
as represented by Eq. (12-17) to achieve typical ambient values. The other
oxidizers in the atmosphere are free radicals. In the lower loop (b) of Fig.
12-4, a second pathway for NO oxidation is shown, with free radicals
participating. These free radicals are derived from the participation of hy-
drocarbons in atmospheric chemical reactions.
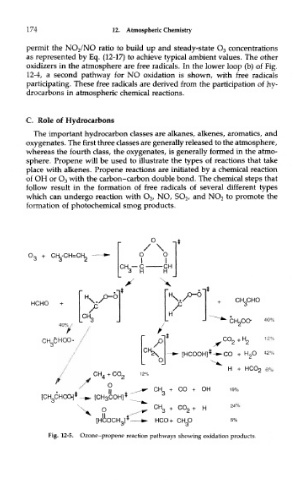
C. Role of Hydrocarbons
The important hydrocarbon classes are alkanes, alkenes, aromatics, and
oxygenates. The first three classes are generally released to the atmosphere,
whereas the fourth class, the oxygenates, is generally formed in the atmo-
sphere. Propene will be used to illustrate the types of reactions that take
place with alkenes. Propene reactions are initiated by a chemical reaction
of OH or O 3 with the carbon-carbon double bond. The chemical steps that
follow result in the formation of free radicals of several different types
which can undergo reaction with O 2, NO, SO 2/ and NO 2 to promote the
formation of photochemical smog products.
Fig. 12-5. Ozone-propene reaction pathways showing oxidation products.