Page 210 - Fundamentals of Air Pollution 3E
P. 210
176 12. Atmospheric Chemistry
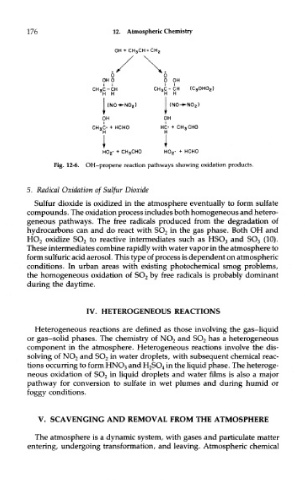
Fig. 12-6. OH-propene reaction pathways showing oxidation products.
5. Radical Oxidation of Sulfur Dioxide
Sulfur dioxide is oxidized in the atmosphere eventually to form sulfate
compounds. The oxidation process includes both homogeneous and hetero-
geneous pathways. The free radicals produced from the degradation of
hydrocarbons can and do react with SO 2 in the gas phase. Both OH and
HO 2 oxidize SO 2 to reactive intermediates such as HSO 3 and SO 3 (10).
These intermediates combine rapidly with water vapor in the atmosphere to
form sulfuric acid aerosol. This type of process is dependent on atmospheric
conditions. In urban areas with existing photochemical smog problems,
the homogeneous oxidation of SO 2 by free radicals is probably dominant
during the daytime.
IV. HETEROGENEOUS REACTIONS
Heterogeneous reactions are defined as those involving the gas-liquid
or gas-solid phases. The chemistry of NO 2 and SO 2 has a heterogeneous
component in the atmosphere. Heterogeneous reactions involve the dis-
solving of NO 2 and SO 2 in water droplets, with subsequent chemical reac-
tions occurring to form HNO 3 and H 2SO 4 in the liquid phase. The heteroge-
neous oxidation of SO 2 in liquid droplets and water films is also a major
pathway for conversion to sulfate in wet plumes and during humid or
foggy conditions.
V. SCAVENGING AND REMOVAL FROM THE ATMOSPHERE
The atmosphere is a dynamic system, with gases and particulate matter
entering, undergoing transformation, and leaving. Atmospheric chemical