Page 137 - Fundamentals of Computational Geoscience Numerical Methods and Algorithms
P. 137
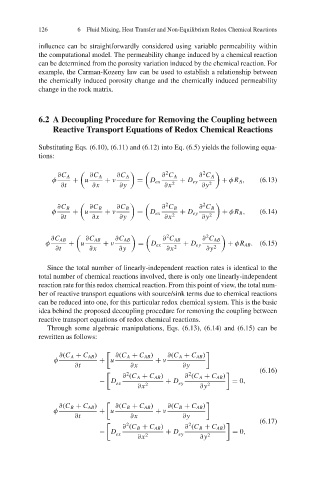
126 6 Fluid Mixing, Heat Transfer and Non-Equilibrium Redox Chemical Reactions
influence can be straightforwardly considered using variable permeability within
the computational model. The permeability change induced by a chemical reaction
can be determined from the porosity variation induced by the chemical reaction. For
example, the Carman-Kozeny law can be used to establish a relationship between
the chemically induced porosity change and the chemically induced permeability
change in the rock matrix.
6.2 A Decoupling Procedure for Removing the Coupling between
Reactive Transport Equations of Redox Chemical Reactions
Substituting Eqs. (6.10), (6.11) and (6.12) into Eq. (6.5) yields the following equa-
tions:
2 2
∂C A ∂C A ∂C A ∂ C A ∂ C A
φ + u + ν = D ex + D ey + φR A , (6.13)
∂t ∂x ∂y ∂x 2 ∂y 2
2 2
∂C B ∂C B ∂C B ∂ C B ∂ C B
φ + u + ν = D ex + D ey + φR B , (6.14)
∂t ∂x ∂y ∂x 2 ∂y 2
2 2
∂C AB ∂C AB ∂C AB ∂ C AB ∂ C AB
φ + u + ν = D ex + D ey + φR AB . (6.15)
∂t ∂x ∂y ∂x 2 ∂y 2
Since the total number of linearly-independent reaction rates is identical to the
total number of chemical reactions involved, there is only one linearly-independent
reaction rate for this redox chemical reaction. From this point of view, the total num-
ber of reactive transport equations with source/sink terms due to chemical reactions
can be reduced into one, for this particular redox chemical system. This is the basic
idea behind the proposed decoupling procedure for removing the coupling between
reactive transport equations of redox chemical reactions.
Through some algebraic manipulations, Eqs. (6.13), (6.14) and (6.15) can be
rewritten as follows:
∂(C A + C AB ) ∂(C A + C AB ) ∂(C A + C AB )
φ + u + ν
∂t ∂x ∂y
(6.16)
2 2
∂ (C A + C AB ) ∂ (C A + C AB )
− D + D = 0,
ex 2 ey 2
∂x ∂y
∂(C B + C AB ) ∂(C B + C AB ) ∂(C B + C AB )
φ + u + ν
∂t ∂x ∂y
(6.17)
∂ (C B + C AB ) ∂ (C B + C AB )
2 2
− D + D = 0,
ex 2 ey 2
∂x ∂y