Page 105 - Fundamentals of Reservoir Engineering
P. 105
PVT ANALYSIS FOR OIL 44
the relationship between surface and reservoir hydrocarbon volumes, equivalent to
equ. (1.25).
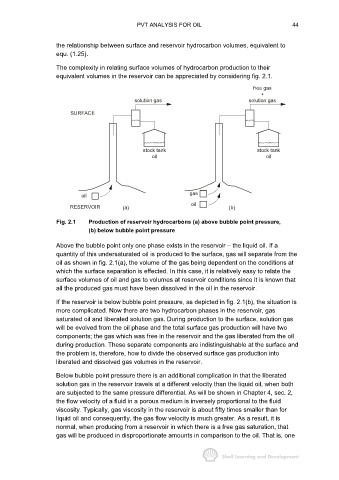
The complexity in relating surface volumes of hydrocarbon production to their
equivalent volumes in the reservoir can be appreciated by considering fig. 2.1.
free gas
+
solution gas solution gas
SURFACE
stock tank stock tank
oil oil
oil gas
oil
RESERVOIR (a) (b)
Fig. 2.1 Production of reservoir hydrocarbons (a) above bubble point pressure,
(b) below bubble point pressure
Above the bubble point only one phase exists in the reservoir − the liquid oil. If a
quantity of this undersaturated oil is produced to the surface, gas will separate from the
oil as shown in fig. 2.1(a), the volume of the gas being dependent on the conditions at
which the surface separation is effected. In this case, it is relatively easy to relate the
surface volumes of oil and gas to volumes at reservoir conditions since it is known that
all the produced gas must have been dissolved in the oil in the reservoir.
If the reservoir is below bubble point pressure, as depicted in fig. 2.1(b), the situation is
more complicated. Now there are two hydrocarbon phases in the reservoir, gas
saturated oil and liberated solution gas. During production to the surface, solution gas
will be evolved from the oil phase and the total surface gas production will have two
components; the gas which was free in the reservoir and the gas liberated from the oil
during production. These separate components are indistinguishable at the surface and
the problem is, therefore, how to divide the observed surface gas production into
liberated and dissolved gas volumes in the reservoir.
Below bubble point pressure there is an additional complication in that the liberated
solution gas in the reservoir travels at a different velocity than the liquid oil, when both
are subjected to the same pressure differential. As will be shown in Chapter 4, sec. 2,
the flow velocity of a fluid in a porous medium is inversely proportional to the fluid
viscosity. Typically, gas viscosity in the reservoir is about fifty times smaller than for
liquid oil and consequently, the gas flow velocity is much greater. As a result, it is
normal, when producing from a reservoir in which there is a free gas saturation, that
gas will be produced in disproportionate amounts in comparison to the oil. That is, one