Page 76 - Fundamentals of Reservoir Engineering
P. 76
SOME BASIC CONCEPTS IN RESERVOIR ENGINEERING 15
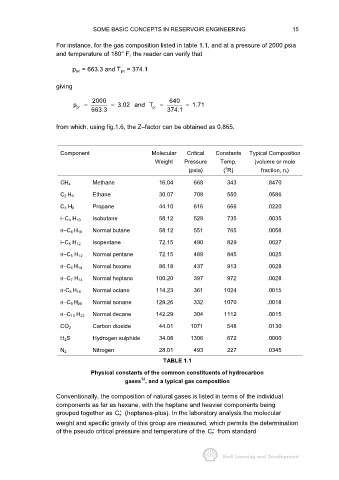
For instance, for the gas composition listed in table 1.1, and at a pressure of 2000 psia
and temperature of 180° F, the reader can verify that
p pc = 663.3 and T pc = 374.1
giving
2000 640
p = = 3.02 and T = = 1.71
pr
663.3 pr 374.1
from which, using fig.1.6, the Z−factor can be obtained as 0.865.
Component Molecular Critical Constants Typical Composition
Weight Pressure Temp. (volume or mole
o
(psia) ( R) fraction, n i )
Methane 16.04 668 343 .8470
CH 4
Ethane 30.07 708 550 .0586
C 2 H 6
Propane 44.10 616 666 .0220
C 3 H 8
Isobutane 58.12 529 735 .0035
i−C 4 H 10
Normal butane 58.12 551 765 .0058
n−C 4 H 10
Isopentane 72.15 490 829 .0027
i−C 5 H 12
Normal pentane 72.15 489 845 .0025
n−C 5 H 12
Normal hexane 86.18 437 913 .0028
n−C 6 H 14
Normal heptane 100.20 397 972 .0028
n−C 7 H 14
Normal octane 114.23 361 1024 .0015
n-C 8 H 18
Normal nonane 128.26 332 1070 .0018
n−C 9 H 20
Normal decane 142.29 304 1112 .0015
n−C 10 H 22
Carbon dioxide 44.01 1071 548 .0130
CO 2
H 2 S Hydrogen sulphide 34.08 1306 672 .0000
Nitrogen 28.01 493 227 .0345
N 2
TABLE 1.1
Physical constants of the common constituents of hydrocarbon
12
gases , and a typical gas composition
Conventionally, the composition of natural gases is listed in terms of the individual
components as far as hexane, with the heptane and heavier components being
grouped together as C (heptanes-plus). In the laboratory analysis the molecular
+
7
weight and specific gravity of this group are measured, which permits the determination
of the pseudo critical pressure and temperature of the C from standard
+
7