Page 802 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 802
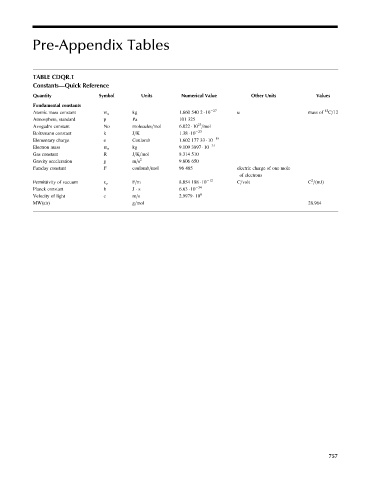
Pre-Appendix Tables
TABLE CDQR.1
Constants—Quick Reference
Quantity Symbol Units Numerical Value Other Units Values
Fundamental constants
Atomic mass constant m u kg 1.660 540 2 10 27 u mass of 12 C=12
Atmosphere, standard p Pa 101 325
23
Avogadro constant No molecules=mol 6.022 10 =mol
Boltzmann constant k J=K 1.38 10 23
19
Elementary charge e Coulomb 1.602 177 33 10
Electron mass m e kg 9.109 3897 10 31
Gas constant R J=K=mol 8.314 510
Gravity acceleration g m=s 2 9.806 650
Faraday constant F coulomb=mol 96 485 electric charge of one mole
of electrons
2
Permittivity of vacuum e o F=m 8.854 188 10 12 C=volt C =(mJ)
Planck constant h J s 6.63 10 34
Velocity of light c m=s 2.9979 10 8
MW(air) g=mol 28.964
757