Page 307 - Gas Purification 5E
P. 307
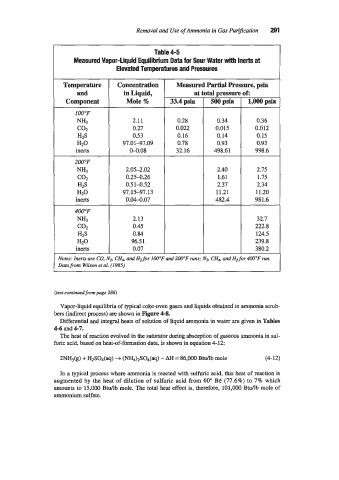
Removal and Use of Ammonia in Gas Purification 291
Table 4-5
Measured Vapor-liquid Equilibrium Data for Sour Water with lnerts at
Elevated Temperatures and Pressures
Temperature Concentration I Measured Partial Pressure, psia
and inLiquid, 1 ital pressure of:
Component Mole % 33.4 psia 500psia I 1,000psia
at
2.11 0.28 0.34 0.36
0.27 0.022 0.015 0.012
0.53 0.16 0.14 0.15
97.01-97.09 0.78 0.93 0.93
04.08 32.16 498.61 998.6
2.05-2.02 2.40 2.75
0.25-0.26 1.61 1.75
0.51-0.52 2.37 2.34
97.15-97.13 11.21 11.20
0.04-0.07 482.4 981.6
I
32.7
222.8
96.5 1 239.8
380.2
Notes: Inerts are CO, N2, CH, and Ht for I00"F and 200°F runs; Nz, CHd. and H2 for 400°F run.
Data from Wilson et aL (1985)
(text continued from page 286)
Vapor-liquid equilibria of typical coke-oven gases and liquids obtained in ammonia scrub-
bers (indirect process) are shown in Figure 4-8.
Differential and integral heats of solution of liquid ammonia in water are given in Tables
4-6 and 47.
The heat of reaction evolved in the saturator during absorption of gaseous ammonia in sul-
furic acid, based on heat-of-formation data, is shown in equation 4-12:
2NH3(g) + HzS04(aq) + (NHJ2S04(aq) - AH = 86,000 BWlb mole (4- 12)
In a typical process where ammonia is reacted with sulfuric acid, this heat of reaction is
augmented by the heat of dilution of sulfuric acid from 60" BB (77.6%) to 7% which
amounts to 15,000 BtuAb mole. The total heat effect is, therefore, 101,000 Btdb mole of
ammonium sulfate.